1000 CLUB OF THE ADSORPTION
Science Citation Index-Expanded (SCI-EXPANDED) --1900-present
There were 53,513,711 publications which included 37,005,526 articles in the SCI-EXPANDED from 1900
“Adsorption”, “sorption”, and “biosorption” were used as the keywords to search titles, abstracts, keywords, and KeyWords Plus.
There were 542,931 publications related to adsorption which included 510,946 articles in SCI-EXPANDED
113 articles with “adsorption”, “sorption”, or “biosorption” in “front page” including titles, abstracts, and author keywords have been cited at least 1000 times from Web of Science Core Collection.
Data last updated 09 August 2019
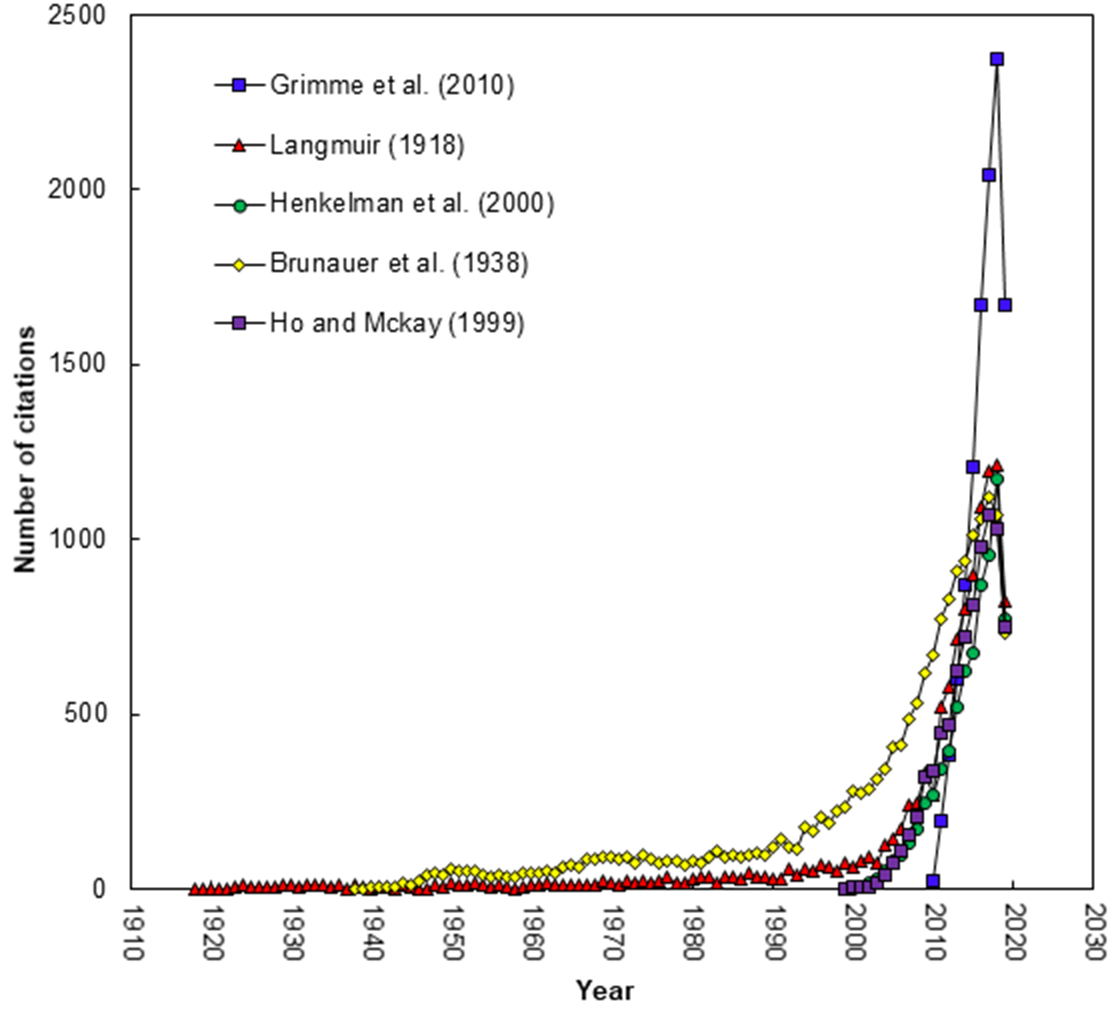
Figure 1. 1000 club of the adsorption by year (TC > 2000)
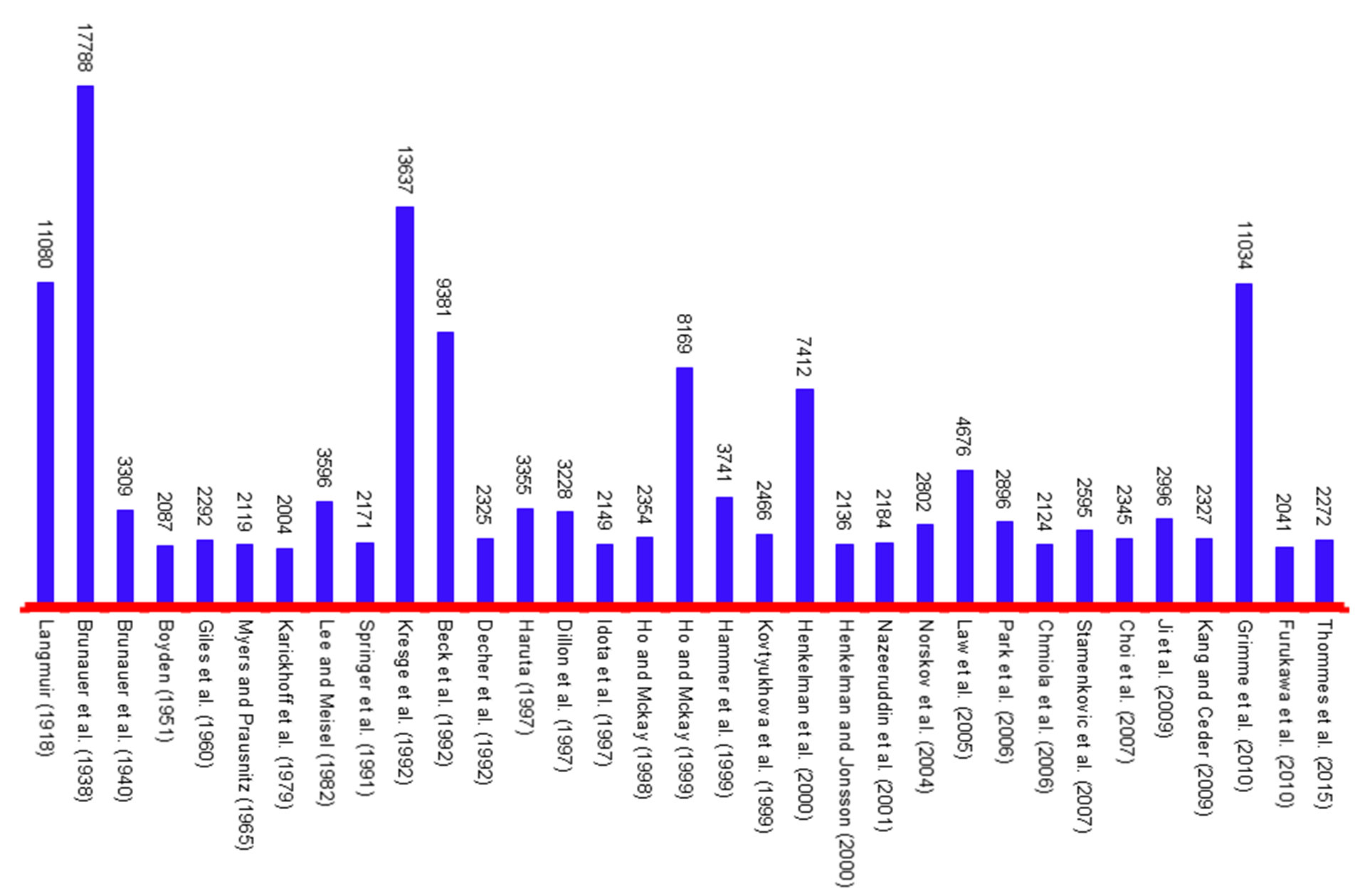
Figure 2. 1000 club of the adsorption by total citations (TC > 2000)
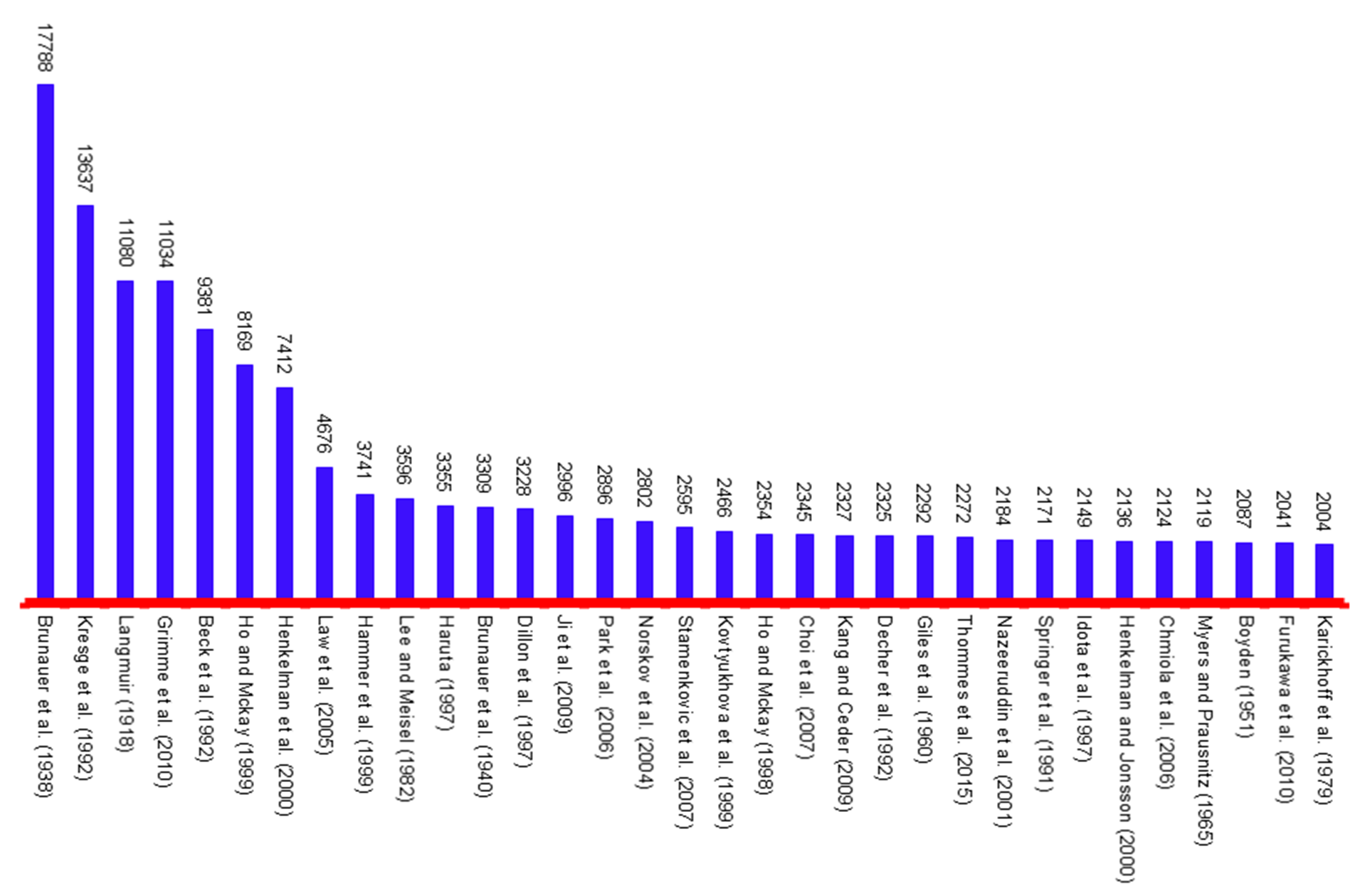
Figure 3. 1000 club of the adsorption by citations per year (TC > 2000)
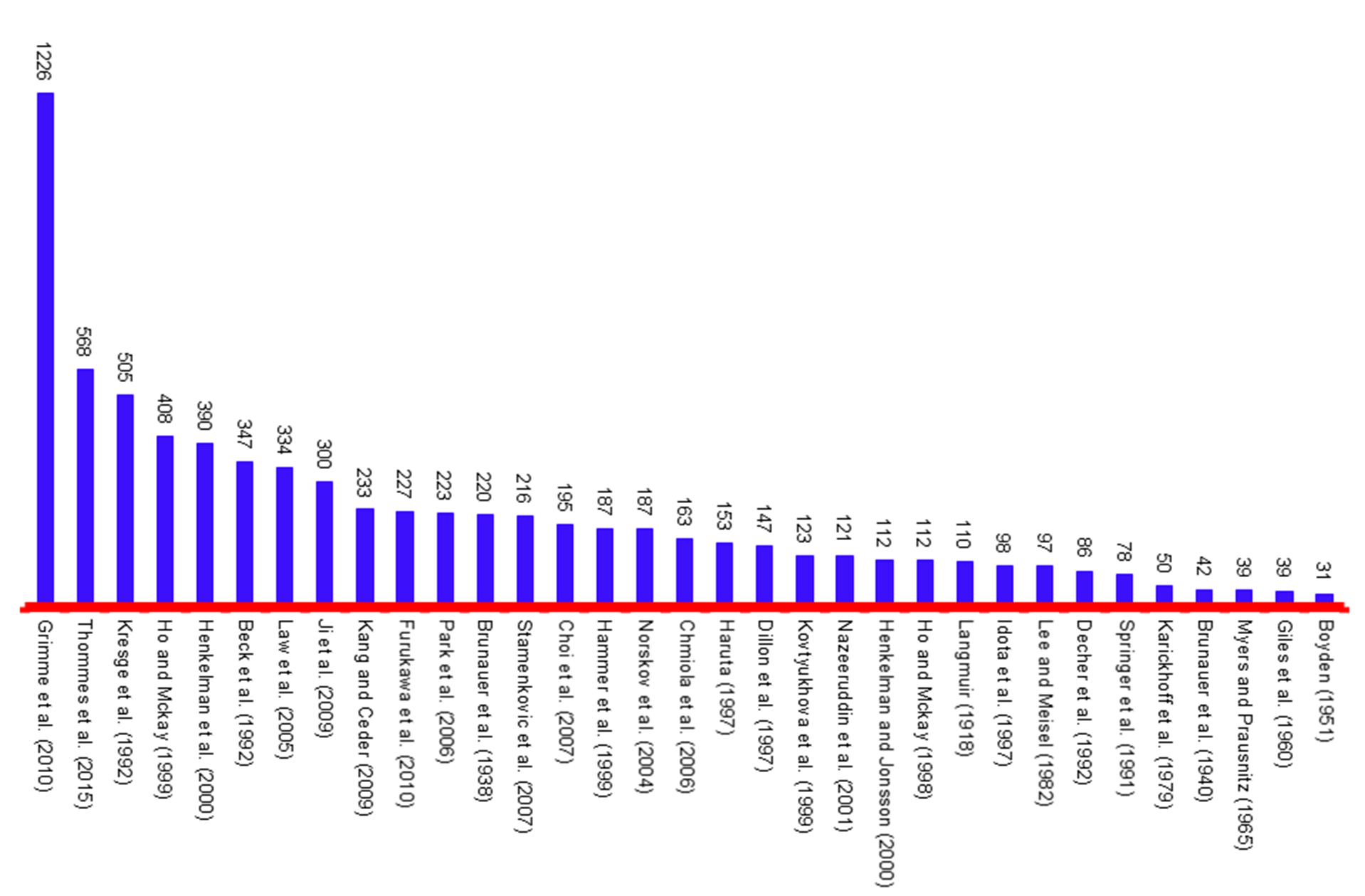
Figure 4. 1000 club of the adsorption by total citations in 2018 (TC > 2000)
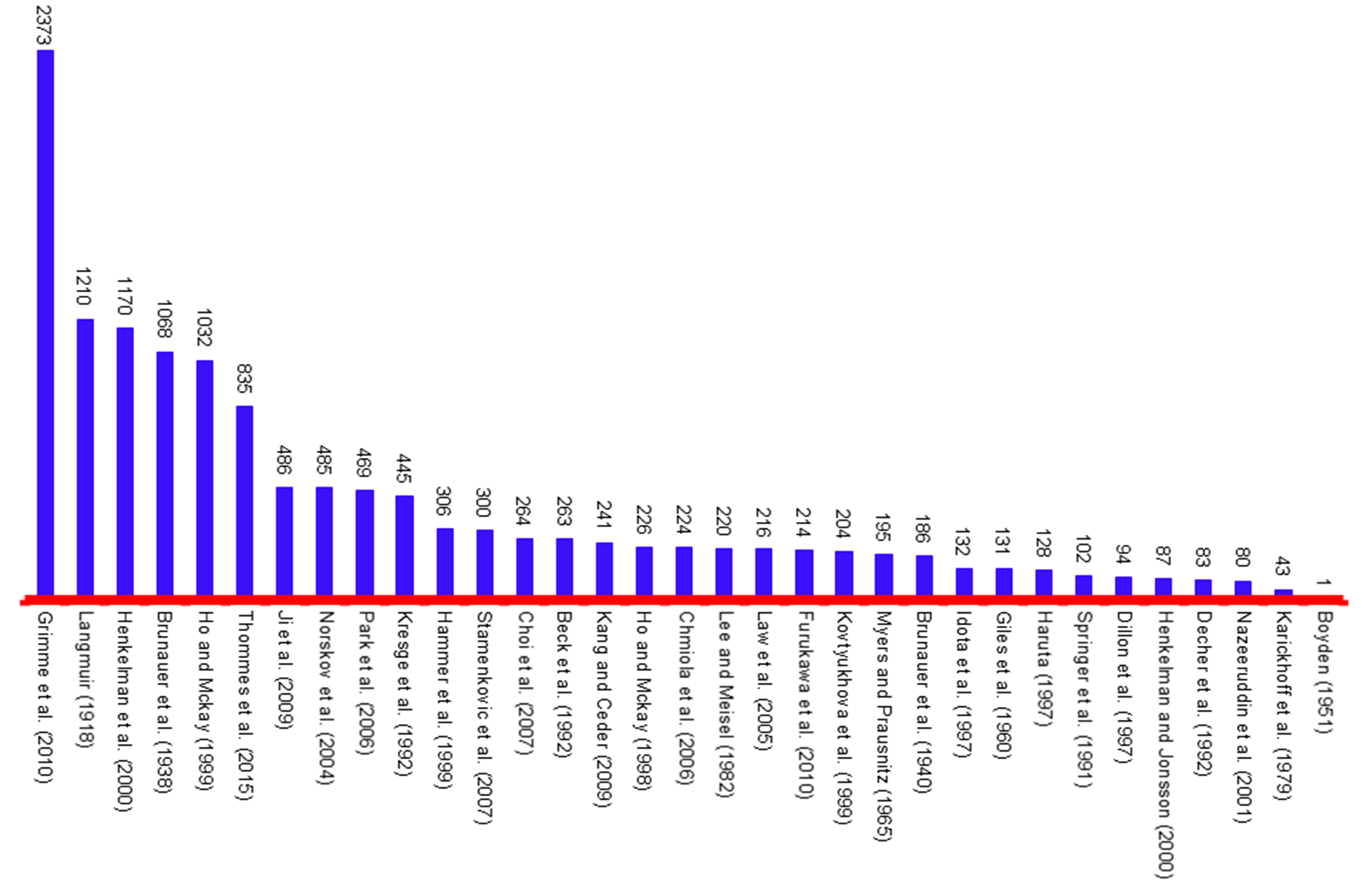
Figure 5. Top articles in 2018 (C2018 > 1000)
|
Stephen Brunauer (Deceased) |
Paul Hugh Emmett (Deceased) |
Edward Teller (Deceased) |
|
1. Brunauer, S., Emmett, P.H. and Teller, E. (1938), Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60 (2), 309-319. |
||
|
Times Cited in Web of Science Core Collection: 17788 |
||
|
Addresses: George Washington University, Bureau of Chemistry and Soils, Washington, DC USA |
||
|
Web of Science Category: Multidisciplinary Chemistry |
||
|
|
|
|
|
|
|
2. Kresge, C.T., Leonowicz, M.E., Roth, W.J., Vartuli, J.C. and Beck, J.S. (1992), Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature, 359 (6397), 710-712. |
||||
|
Times Cited in Web of Science Core Collection: 13637 |
||||
|
KeyWords Plus: Phosphate |
||||
|
Abstract: MICROPOROUS and mesoporous inorganic solids (with pore diameters of less-than-or-equal-to 20 angstrom and approximately 20-500 angstrom respectively)1 have found great utility as catalysts and sorption media because of their large internal surface area. Typical microporous materials are the crystalline framework solids, such as zeolites2, but the largest pore dimensions found so far are approximately 10-12 angstrom for some metallophosphates3-5 and approximately 14 angstrom for the mineral cacoxenite6. Examples of mesoporous solids include silicas7 and modified layered materials8-11, but these are invariably amorphous or paracrystalline, with pores that are irregularly spaced and broadly distributed in size8,12. Pore size can be controlled by intercalation of layered silicates with a surfactant species9,13, but the final product retains, in part, the layered nature of the precursor material. Here we report the synthesis of mesoporous solids from the calcination of aluminosilicate gels in the presence of surfactants. The material14,15 possesses regular arrays of uniform channels, the dimensions of which can be tailored (in the range 16 angstrom to 100 angstrom or more) through the choice of surfactant, auxiliary chemicals and reaction conditions. We propose that the formation of these materials takes place by means of a liquid-crystal 'templating' mechanism, in which the silicate material forms inorganic walls between ordered surfactant micelles. |
||||
|
Addresses: Mobil Research and Development Corporation, Central Research Laboratory, Princeton, NJ 08540, USA; Central Research Laboratory, Princeton, NJ 08543, USA |
||||
|
Present addresses: Charles T. Kresge: M.E. Leonowicz: W.J. Roth: J.C. Vartuli: J.S. Beck: |
||||
|
Reprint Address: Charles T. Kresge, Mobil Research and Development Corporation, Paulsboro Research Laboratory, Paulsboro, NJ 08066, USA |
||||
|
Web of Science Category: Multidisciplinary Sciences |
||||
|
Irving Langmuir (Deceased) |
|
3. Langmuir, I. (1918), The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361-1403. |
|
Times Cited in Web of Science Core Collection: 11080 |
|
Addresses: Research Laboratory of the General Electric Co., NY USA |
|
Web of Science Category: Chemistry, Multidisciplinary |
|
Leibniz Prize 2015 |
|
|
|
|
4. Grimme, S., Antony, J., Ehrlich, S. and Krieg, H. (2010), A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Journal of Chemical Physics, 132 (15), Article Number: 154104. |
|||
|
Times Cited in Web of Science Core Collection: 11034 |
|||
|
Abstract: The method of dispersion correction as an add-on to standard Kohn-Sham density functional theory (DFT-D) has been refined regarding higher accuracy, broader range of applicability, and less empiricism. The main new ingredients are atom-pairwise specific dispersion coefficients and cutoff radii that are both computed from first principles. The coefficients for new eighth-order dispersion terms are computed using established recursion relations. System (geometry) dependent information is used for the first time in a DFT-D type approach by employing the new concept of fractional coordination numbers (CN). They are used to interpolate between dispersion coefficients of atoms in different chemical environments. The method only requires adjustment of two global parameters for each density functional, is asymptotically exact for a gas of weakly interacting neutral atoms, and easily allows the computation of atomic forces. Three-body nonadditivity terms are considered. The method has been assessed on standard benchmark sets for inter- and intramolecular noncovalent interactions with a particular emphasis on a consistent description of light and heavy element systems. The mean absolute deviations for the S22 benchmark set of noncovalent interactions for 11 standard density functionals decrease by 15%-40% compared to the previous (already accurate) DFT-D version. Spectacular improvements are found for a tripeptide-folding model and all tested metallic systems. The rectification of the long-range behavior and the use of more accurate C-6 coefficients also lead to a much better description of large (infinite) systems as shown for graphene sheets and the adsorption of benzene on an Ag(111) surface. For graphene it is found that the inclusion of three-body terms substantially (by about 10%) weakens the interlayer binding. We propose the revised DFT-D method as a general tool for the computation of the dispersion energy in molecules and solids of any kind with DFT and related (low-cost) electronic structure methods for large systems. |
|||
|
Addresses: Stefan Grimme: Theoretische Organische Chemie, Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, D-48149 Münster, Germany Jens Antony: Theoretische Organische Chemie, Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, D-48149 Münster, Germany, E-mail: jens.antony@uni-muenster.de Stephan Ehrlich: Theoretische Organische Chemie, Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, D-48149 Münster, Germany, E-mail: stephan.ehrlich@uni-muenster.de Helge Krieg: Theoretische Organische Chemie, Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, D-48149 Münster, Germany, E-mail: hkrieg@uni-muenster.de |
|||
|
Reprint Address: Stefan Grimme: Theoretische Organische Chemie, Organisch-Chemisches Institut, Universität Münster, Corrensstrasse 40, D-48149 Münster, Germany. E-mail: grimmes@uni-muenster.de |
|||
|
Web of Science Category: Atomic Molecular & Chemical Physics |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5. Beck, J.S., Vartuli, J.C., Roth, W.J., Leonowicz, M.E., Kresge, C.T., Schmitt, K.D., Chu, C.T.W., Olson, D.H., Sheppard, E.W., McCullen, S.B., Higgins, J.B. and Schlenker, J.L. (1992), A new family of mesoporous molecular-sieves prepared with liquid crystal templates. Journal of the American Chemical Society, 114 (27), 10834-10843. |
|||||
|
Times Cited in Web of Science Core Collection: 9381 |
|||||
|
Abstract: The synthesis, characterization, and proposed mechanism of formation of a new family of silicate/aluminosilicate mesoporous molecular sieves designated as M41S is described. MCM-41, one member of this family, exhibits a hexagonal arrangement of uniform mesopores whose dimensions may be engineered in the range of approximately 15 angstrom to greater than 100 angstrom. Other members of this family, including a material exhibiting cubic symmetry, have been synthesized. The larger pore M41S materials typically have surface areas above 700 m2/g and hydrocarbon sorption capacities of 0.7 cc/g and greater. A templating mechanism (liquid crystal templating-LCT) in which surfactant liquid crystal structures serve as organic templates is proposed for the formation of these materials. In support of this templating mechanism, it was demonstrated that the structure and pore dimensions of MCM-41 materials are intimately linked to the properties of the surfactant, including surfactant chain length and solution chemistry. The presence of variable pore size MCM-41, cubic material, and other phases indicates that M41S is an extensive family of materials. |
|||||
|
Addresses: Mobil Research and Development Corporation, Paulsboro Research Laboratory, Princeton, NJ 08543; Mobil Research and Development Corporation, Paulsboro Research Laboratory, Paulsboro, NJ 08066 |
|||||
|
Present addresses: J.S. Beck: J.C. Vartuli: W.J. Roth: M.E. Leonowicz: C.T. Kresge: K.D. Schmitt: C.T.W. Chu: D.H. Olson: E.W. Sheppard: S.B. Mccullen: J.B. Higgins: J.L. Schlenker: |
|||||
|
Reprint Address: Beck, JS, Mobil Research and Development Corporation, Paulsboro Research Laboratory, Princeton, NJ 08543. |
|||||
|
Web of Science Category: Multidisciplinary Chemistry |
|||||
|
|
|
|
6. Ho, Y.S. and McKay, G. (1999), Pseudo-second order model for sorption processes. Process Biochemistry, 34 (5), 451-465. |
|
|
Times Cited in Web of Science Core Collection: 8169 |
|
|
Author Keywords: Kinetics; Sorption; Pseudo-Second Order |
|
|
Abstract: A literature review of the use of sorbents and biosorbents to treat polluted aqueous effluents containing dyes/organics or metal ions has been conducted. Over 70 systems have been reported since 1984 and over 43 of these reported the mechanism as being a pseudo-first order kinetic mechanism. Three sorption kinetic models are presented in this paper and have been used to test 11 of the literature systems previously reported as first order kinetics and one system previously reported as a second order process. In all 12 systems, the highest correlation coefficients were obtained for the pseudo-second order kinetic model. (C) 1999 Elsevier Science Ireland Ltd. All rights reserved. |
|
|
Addresses: Hong Kong University Science & Technology, Department of Chemical Engineering, Hong Kong, People’s R China |
|
|
Present addresses: Yuh-Shan Ho: Asia University, Water Research Centre, Taichung 41354, Taiwan. E-mail: ysho@asia.edu.tw Gordon McKay: Hamad Bin Khalifa University, Sustainable Development, College of Science & Engineering, E-mail: gmckay@qf.org.qa |
|
|
Reprint Address: McKay, G, Hong Kong University Science & Technology, Department of Chemical Engineering, Hong Kong, People’s R China |
|
|
Web of Science Category: Biochemistry & Molecular Biology; Biotechnology & Applied Microbiology; Chemical Engineering |
|
|
|
|
|
|
7. Henkelman, G., Uberuaga, B.P. and Jónsson, H. (2000), A climbing image nudged elastic band method for finding saddle points and minimum energy paths. Journal of Chemical Physics, 113 (22), 9901-9904. |
||
|
Times Cited in Web of Science Core Collection: 7412 |
||
|
Abstract: A modification of the nudged elastic band method for finding minimum energy paths is presented. One of the images is made to climb up along the elastic band to converge rigorously on the highest saddle point. Also, variable spring constants are used to increase the density of images near the top of the energy barrier to get an improved estimate of the reaction coordinate near the saddle point. Applications to CH4 dissociative adsorption on Ir(111) and H-2 on Si(100) using plane wave based density functional theory are presented. (C) 2000 American Institute of Physics. [S0021-9606(00)71246-3]. |
||
|
Addresses: University of Washington, Department of Chemistry 351700, Seattle, WA 98195 USA; University of Washington, Department of Physics 351560, Seattle, WA 98195 USA |
||
|
Present addresses: Graeme Henkelman: University of Texas Austin, Department of Chemistry & Biochemistry, Austin, TX 78712 USA. E-mail: henkelman@mail.utexas.edu Blas Pedro Uberuaga: Technical Staff Member, Materials Science and Technology Division (MST-8) Los Alamos National Laboratory, USA. E-mail: blas@lanl.gov Hannes Jónsson: University of Iceland, Chemistry division of the Science Institute, Iceland; E-mail: hj@hi.is |
||
|
Reprint Address: Henkelman, G, University of Washington, Department of Chemistry 351700, Seattle, WA 98195 USA. |
||
|
Web of Science Category: Atomic, Molecular & Chemical Physics |
||
|
|
|
|
|
|
|
8. Law, M., Greene, L.E., Johnson, J.C., Saykally, R. and Yang, P.D. (2005), Nanowire dye-sensitized solar cells. Nature Materials, 4 (6), 455-459. |
||||
|
Times Cited in Web of Science Core Collection: 4676 |
||||
|
Author Keywords: desulfurization; gasoline; fuels; diesel fuel; jet fuel; catalysis; adsorption |
||||
|
Abstract: Excitonic solar cells(1)-including organic, hybrid organic inorganic and dye-sensitized cells (DSCs)-are promising devices for inexpensive, large-scale solar energy conversion. The DSC is currently the most efficient(2) and stable(3) excitonic photocell. Central to this device is a thick nanoparticle film that provides a large surface area for the adsorption of light-harvesting molecules. However, nanoparticle DSCs rely on trap-limited diffusion for electron transport, a slow mechanism that can limit device efficiency, especially at longer wavelengths. Here we introduce a version of the dye-sensitized cell in which the traditional nanoparticle film is replaced by a dense array of oriented, crystalline ZnO nanowires. The nanowire anode is synthesized by mild aqueous chemistry and features a surface area up to one-fifth as large as a nanoparticle cell. The direct electrical pathways provided by the nanowires ensure the rapid collection of carriers generated throughout the device, and a full Sun efficiency of 1.5% is demonstrated, limited primarily by the surface area of the nanowire array. |
||||
|
Addresses: University of California, Berkeley, Department of Chemistry, Berkeley, CA 94720 USA; University of California, Berkeley, Lawrence Berkeley Laboratory, Materials Science Division, Berkeley, CA 94720 USA |
||||
|
Present addresses: Matt Law: University of California, Irvine, Department of Chemistry, 2127 Natural Sciences II, Irvine, CA 92697 USA; E-mail: matt.law@uci.edu Lori E. Greene: University of California, Irvine, School of Physical Sciences, USA; E-mail: legreene@uci.edu Justin C. Johnson: National Renewable Energy Laboratory, Chemical and Materials Science Center, USA; E-mail: Justin.Johnson@nrel.gov Richard Saykally: University of California, Berkeley, Department of Chemistry, Berkeley, CA 94720 USA. E-mail: saykally@berkeley.edu Peidong Yang: University of California, Berkeley, Department of Chemistry, Berkeley, CA 94720 USA. E-mail: p_yang@berkeley.edu |
||||
|
Reprint Address: Law, M, University of California, Berkeley, Department of Chemistry, Berkeley, CA 94720 USA. |
||||
|
Web of Science Category: Physical Chemistry; Materials Science, Multidisciplinary; Applied Physics; Condensed Matter Physics |
||||
|
|
|
|
|
9. Hammer, B., Hansen, L.B. and Nørskov, J.K. (1999), Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Physical Review B, 59 (11), 7413-7421. |
||
|
Times Cited in Web of Science Core Collection: 3741 |
||
|
Abstract: A simple formulation of a generalized gradient approximation for the exchange and correlation energy of electrons has been proposed by Perdew, Burke, and Ernzerhof (PBE) [Phys. Rev. Lett. 77, 3865 (1996)]. Subsequently Zhang and Yang [Phys. Rev. Lett. 80, 890 (1998)] have shown that a slight revision of the PBE functional systematically improves the atomization energies for a large database of small molecules. In the present work, we show that the Zhang and Yang functional (revPBE) also improves the chemisorption energetics of atoms and molecules on transition-metal surfaces. Our test systems comprise atomic and molecular adsorption of oxygen, CO, and NO on Ni(100), Ni(111), Rh(100), Pd(100), and Pd(111) surfaces. As the revPBE functional may locally violate the Lieb-Oxford criterion, we further develop an alternative revision of the PBE functional, RPBE, which gives the same improvement of the chemisorption energies as the revPBE functional at the same time as it fulfills the Lieb-Oxford criterion locally. [S0163-1829(99)02711-3]. |
||
|
Addresses: University of Aalborg, Institute of Physics, DK-9220 Aalborg, Denmark; Technical University of Denmark, Department of Physics, Center for Atomic-scale Materials Physics, DK-2800 Lyngby, Denmark |
||
|
Present addresses: Bjørk Hammer: University of Aalborg, Institute of Physics, Pontoppidanstr 103, DK-9220 Aalborg, Denmark L.B. Hansen: Jens K. Nørskov: |
||
|
Reprint Address: Hammer, B, University of Aalborg, Institute of Physics, Pontoppidanstr 103, DK-9220 Aalborg, Denmark |
||
|
Web of Science Category: Condensed Matter Physics |
||
|
|
|
|
10. Lee, P.C. and Meisel, D. (1982), Adsorption and surface-enhanced Raman of dyes on silver and gold sols. Journal of Physical Chemistry, 86 (17), 3391-3395. |
|
|
Times Cited in Web of Science Core Collection: 3596 |
|
|
Addresses: Argonne National Laboratory, Chemistry Division, Argonne, IL 60439, USA |
|
|
Present addresses: P.C. Lee: Dan Meisel: Radiation Laboratory and Department of Chemistry & Biochemistry, University of Notre Dame, Notre Dame, IN 46556, USA. E-mail: dani@nd.edu |
|
|
Reprint Address: Meisel, D, Argonne National Laboratory, Chemistry Division, Argonne, IL 60439, USA |
|
|
Web of Science Category: Physical Chemistry |
|
|
|
|
11. Haruta, M. (1997), Size- and support-dependency in the catalysis of gold. Catalysis Today, 36 (1), 153-166. |
|
Times Cited in Web of Science Core Collection: 3355 |
|
Author Keywords: Gold Catalysts; Adsorption; Preparation |
|
Abstract: The adsorption properties and reactivities of gold are summarized in terms of their size dependency from bulk to fine particles, clusters and atoms. The catalytic performances of gold markedly depend on dispersion, supports, and preparation methods. When gold is deposited on select metal oxides as hemispherical ultra-fine particles with diameters smaller than 5 nn, it exhibits surprisingly high activities and/or selectivities in the combustion of CO and saturated hydrocarbons, the oxidation-decomposition of amines and organic halogenated compounds, the partial oxidation of hydrocarbons, the hydrogenation of carbon oxides, unsaturated carbonyl compounds, alkynes and alkadienes, and the reduction of nitrogen oxides. The unique catalytic nature of supported gold can be explained by assuming that the gold-metal oxide perimeter interface acts as a site for activating at least one of the reactants, for example, oxygen. Some examples and future prospects in applications are also briefly described. |
|
Addresses: Government Industrial Research Institute of Osaka, Midorigaoka I, Ikeda 563, Japan; Kishida Chemicals Company, Ltd, Joshoji-machi, Kadoma 571, Japan; Research Development Corporation of Japan, Science Building, 5-2 Nagata-Cho 2-chome, Tokyo 100 Japan |
|
Present addresses: Masatake Haruta: Department of Applied Chemistry, Graduate School of Urban Environmental Sciences, Tokyo Metropolitan University, 1-1 Minami-osawa, Hachioji, Tokyo 192-0397, Japan; E-mail: haruta-masatake@center.tmu.ac.jp |
|
Reprint Address: Haruta, M (reprint author), Government Industrial Research Institute of Osaka, Midorigaoka I, Ikeda 563, Japan |
|
Web of Science Category: Applied Chemistry; Physical Chemistry; Chemical Engineering |
|
|
|
|
|
|
12. Brunauer, S., Deming, L.S., Deming, W.E. and Teller, R. (1940), On a theory of the van der Waals adsorption of gases. Journal of the American Chemical Society, 62 (7), 1723-1732. |
|||
|
Times Cited in Web of Science Core Collection: 3309 |
|||
|
Addresses: Bureau of Agricultural Chemistry and Engineering, George Washington University |
|||
|
Web of Science Category: Multidisciplinary Chemistry |
|||
|
|
|
|
|
|
|
|
13. Dillon, A.C., Jones, K.M., Bekkedahl, T.A., Kiang, C.H., Bethune, D.S. and Heben, M.J. (1997), Storage of hydrogen in single-walled carbon nanotubes. Nature, 386 (6623), 377-379. |
|||||
|
Times Cited in Web of Science Core Collection: 3228 |
|||||
|
Abstract: Pores of molecular dimensions can adsorb large quantities of gases owing to the enhanced density of the adsorbed material inside the pores(1), a consequence of the attractive potential of the pore walls, Pederson and Broughton have suggested(2) that carbon nanotubes, which have diameters of typically a few nanometres, should be able to draw up liquids by capillarity, and this effect has been seen for low-surface-tension liquids in large-diameter, multi-walled nanotubes(3). Here we show that a gas can condense to high density inside narrow, single-walled nanotubes (SWNTs), Temperature-programmed desorption spectrosocopy shows that hydrogen will condense inside SWNTs under conditions that do not induce adsorption within a standard mesoporous activated carbon, The very high hydrogen uptake in these materials suggests that they might be effective as a hydrogen-storage material for fuel-cell electric vehicles. |
|||||
|
Addresses: National Renewable Energy Laboratory, Golden, CO 80401, USA; IBM Research Division, Almaden Research Center, San Jose, CA 95120, USA |
|||||
|
Present addresses: Anne C. Dillon: National Renewable Energy Laboratory, Center for Materials and Chemical Sciences, 1617 Cole Boulevard, Golden, CO 80401 USA. E-mail: anne.dillon@nrel.gov Kim M. Jones: National Renewable Energy Laboratory, National Center for Photovoltaics, Golden, CO 80401 USA T.A. Bekkedahl: C.H. Kiang: D.S. Bethune: Michael J. Heben: Chemical & Materials Science Center, National Renewable Energy Laboratory, 1617 Cole Boulevard, Golden, Colorado 80401 USA, Department of Physics and Astronomy, The University of Toledo, Toledo, Ohio 43606 USA. E-mail: mheben@utnet.utoledo.edu |
|||||
|
Web of Science Category: Multidisciplinary Sciences |
|||||
|
|
Kyu Tae Lee |
|
|
14. Ji, X.L., Lee, K.T. and Nazar, L.F. (2009), A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials, 8 (6), 500-506. |
||
|
Times Cited in Web of Science Core Collection: 2996 |
||
|
Abstract: The Li-S battery has been under intense scrutiny for over two decades, as it offers the possibility of high gravimetric capacities and theoretical energy densities ranging up to a factor of five beyond conventional Li-ion systems. Herein, we report the feasibility to approach such capacities by creating highly ordered interwoven composites. The conductive mesoporous carbon framework precisely constrains sulphur nanofiller growth within its channels and generates essential electrical contact to the insulating sulphur. The structure provides access to Li(+) ingress/egress for reactivity with the sulphur, and we speculate that the kinetic inhibition to diffusion within the framework and the sorption properties of the carbon aid in trapping the polysulphides formed during redox. Polymer modification of the carbon surface further provides a chemical gradient that retards diffusion of these large anions out of the electrode, thus facilitating more complete reaction. Reversible capacities up to 1,320m Ah g(-1) are attained. The assembly process is simple and broadly applicable, conceptually providing new opportunities for materials scientists for tailored design that can be extended to many different electrode materials. |
||
|
Addresses: University of Waterloo, Department of Chemistry, Waterloo, Ontario N2L 3G1, Canada |
||
|
Present addresses: Xiulei Ji: Departmenet of Chemistry, Oregon State University, Corvallis, OR, 97331, USA. E-mail: david.ji@oregonstate.edu Kyu Tae Lee: Ulsan National Institute of Science and Technology, Urusan, Ulsan, South Korea. E-mail: ktlee@unist.ac.kr Linda F. Nazar: Department of Chemistry, University of Waterloo, 200 University Avenue West, Waterloo, Ontario, N2L 3G1 Canada. E-mail: lfnazar@uwaterloo.ca |
||
|
Reprint Address: Linda F. Nazar (reprint author) University of Waterloo, Department of Chemistry, Waterloo, Ontario N2L 3G1, Canada. E-mail: lfnazar@uwaterloo.ca |
||
|
Web of Science Category: Physical Chemistry; Multidisciplinary Materials Science; Applied Physics; Condensed Matter Physics |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
15. Park, K.S., Ni, Z., Cote, A.P., Choi, J.Y., Huang, R.D., Uribe-Romo, F.J., Chae, H.K., O’Keeffe, M. and Yaghi, O.M. (2006), Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proceedings of the National Academy of Sciences of the United States of America, 103 (27), 10186-10191. |
||||
|
Times Cited in Web of Science Core Collection: 2896 |
||||
|
Abstract: Twelve zeolitic imidazolate frameworks (ZIFs; termed ZIF-1 to -12) have been synthesized as crystals by copolymerization of either Zn(II) (ZIF-1 to -4, -6 to -8, and -10 to -11) or Co(II) (ZIF-9 and -12) with imidazolate-type links. The ZIF crystal structures are based on the nets of seven distinct aluminosilicate zeolites: tetrahedral Si(AI) and the bridging O are replaced with transition metal ion and imidazolate link, respectively. In addition, one example of mixed-coordination imidazolate of Zn(II) and In(III) (ZIF-5) based on the garnet net is reported. Study of the gas adsorption and thermal and chemical stability of two prototypical members, ZIF-8 and -11, demonstrated their permanent porosity (Langmuir surface area = 1,810 m(2)/g), high thermal stability (up to 550 degrees C), and remarkable chemical resistance to boiling alkaline water and organic solvents. |
||||
|
Addresses: Department of Chemistry and Biochemistry, Center for Reticular Materials Research at California NanoSystems Institute, University of California, Los Angeles, CA 90095 USA. Department of Chemistry Education, Seoul National University, Seoul 151-748, South Korea. Institute for Chemical Physics, School of Science, Beijing Institute of Technology, Beijing 100081, Peoples R China. Department of Chemistry, Arizona State University, Tempe, AZ 85287 USA. |
||||
|
Present addresses: Kyo Sung Park: Zheng Ni: Adrien P. Côté: Jae Yong Choi: Rudan Huang: Fernando J. Uribe-Romo: Hee K. Chae: Michael O’Keeffe: Department of Chemistry, Arizona State University, Tempe, AZ 85287 USA. E-mail: mokeeffe@asu.edu Omar M. Yaghi: E-mail: yaghi@berkeley.edu |
||||
|
Reprint Address: Yaghi, OM (reprint author), Department of Chemistry and Biochemistry, Center for Reticular Materials Research at California NanoSystems Institute, University of California, Los Angeles, CA 90095 USA. E-mail: yaghi@chem.ucla.edu |
||||
|
Web of Science Category: Multidisciplinary Sciences |
||||
|
|
|
|
|
|
|
|
|
|
|
16. Nørskov, J.K., Rossmeisl, J., Logadottir, A., Lindqvist, L., Kitchin, J.R., Bligaard, T. and Jónsson, H. (2004), Origin of the overpotential for oxygen reduction at a fuel-cell cathode. Journal of Physical Chemistry B, 108 (46), 17886-17892. |
|||
|
Times Cited in Web of Science Core Collection: 2802 |
|||
|
Author Keywords: |
|||
|
Abstract: We present a method for calculating the stability of reaction intermediates of electrochemical processes on the basis of electronic structure calculations. We used that method in combination with detailed density functional calculations to develop a detailed description of the free-energy landscape of the electrochemical oxygen reduction reaction over Pt(111) as a function of applied bias. This allowed us to identify the origin of the overpotential found for this reaction. Adsorbed oxygen and hydroxyl are found to be very stable intermediates at potentials close to equilibrium, and the calculated rate constant for the activated proton/electron transfer to adsorbed oxygen or hydroxyl can account quantitatively for the observed kinetics. On the basis of a database of calculated oxygen and hydroxyl adsorption energies, the trends in the oxygen reduction rate for a large number of different transition and noble metals can be accounted for. Alternative reaction mechanisms involving proton/electron transfer to adsorbed molecular oxygen were also considered, and this peroxide mechanism was found to dominate for the most noble metals. The model suggests ways to improve the electrocatalytic properties of fuel-cell cathodes. |
|||
|
Addresses: Center for Atomic-scale Materials Physics, Department of Physics, Technical University of Denmark, DK-2800 Lyngby, Denmark Department of Chemical Engineering, University of Delaware, Newark, Delaware 19716 Science Institute, VR-II, University of Iceland, IS-107 Reykjavík, Iceland Faculty of Science, VR-II, University of Iceland, IS-107 Reykjavík, Iceland |
|||
|
Present addresses: Jens K. Nørskov: Stanford University, Palo Alto, California, USA. E-mail: norskov@stanford.edu Jan Rossmeisl: Technical University of Denmark, Lyngby, Capital Region, Denmark. E-mail: jross@fysik.dtu.dk Áshildur Logadóttir: Technical University of Denmark, Lyngby, Capital Region, Denmark L Lindqvist: Technical University of Denmark, Copenhagen, Capital Region, Denmark John R. Kitchin: Department of Chemical Engineering, Carnegie Mellon University, Pittsburgh, USA. E-mail: jkitchin@andrew.cmu.edu Thomas Bligaard: Stanford University, SLAC National Accelerator Laboratory, USA. E-mail: bligaard@stanford.edu Hannes Jónsson: University of Iceland, Chemistry division of the Science Institute, Iceland; E-mail: hj@hi.is |
|||
|
Reprint Address: J.K. Nørskov (reprint author), Center for Atomic-scale Materials Physics, Department of Physics, Technical University of Denmark, DK-2800 Lyngby, Denmark. E-mail Addresseses: norskov@fysik.dtu.dk |
|||
|
Web of Science Category: Chemistry, Physical |
|||
|
|
|
|
|
|
|
|
|
|
|
17. Stamenkovic, V.R., Fowler, B., Mun, B.S., Wang, G.F., Ross, P.N., Lucas, C.A. and Marković, N.M. (2007), Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science, 315 (5811), 493-497. |
|||
|
Times Cited in Web of Science Core Collection: 2595 |
|||
|
Abstract: The slow rate of the oxygen reduction reaction (ORR) in the polymer electrolyte membrane fuel cell ( PEMFC) is the main limitation for automotive applications. We demonstrated that the Pt3Ni( 111) surface is 10-fold more active for the ORR than the corresponding Pt(111) surface and 90-fold more active than the current state-of-the- art Pt/C catalysts for PEMFC. The Pt3Ni( 111) surface has an unusual electronic structure (d-band center position) and arrangement of surface atoms in the near-surface region. Under operating conditions relevant to fuel cells, its near-surface layer exhibits a highly structured compositional oscillation in the outermost and third layers, which are Pt-rich, and in the second atomic layer, which is Ni-rich. The weak interaction between the Pt surface atoms and nonreactive oxygenated species increases the number of active sites for O-2 adsorption. |
|||
|
Addresses: Argonne Natl Lab, Div Mat Sci, Argonne, IL 60439 USA. Univ Calif Berkeley, Lawrence Berkeley Lab, Div Sci Mat, Berkeley, CA 94720 USA. Univ Liverpool, Dept Phys, Oliver Lodge Lab, Liverpool L69 7ZE, Merseyside, England. Univ S Carolina, Dept Chem & Phys, Aiken, SC 29801 USA. |
|||
|
Present addresses: Vojislav R. Stamenkovic: Materials Science Division, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: vrstamenkovic@anl.gov Ben Fowler: Oliver Lodge Laboratory, Department of Physics, University of Liverpool, Liverpool, L69 7ZE, UK. Bongjin Simon Mun: Materials Sciences Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720, USA. Guofeng Wang: Department of Chemistry and Physics, University of South Carolina, Aiken, SC 29801, USA. Philip N. Ross: Materials Sciences Division, Lawrence Berkeley National Laboratory, University of California, Berkeley, CA 94720, USA. E-mail: PNRoss@lbl.gov Christopher A. Lucas: Oliver Lodge Laboratory, Department of Physics, University of Liverpool, Liverpool, L69 7ZE, UK. E-mail: Clucas@liverpool.ac.uk Nenad M. Marković: Materials Science Division, Argonne National Laboratory, Argonne, IL 60439, USA. E-mail: nmmarkovic@anl.gov |
|||
|
Reprint Address: Stamenkovic, VR. Argonne Natl Lab, Div Mat Sci, 9700 S Cass Ave, Argonne, IL 60439 USA. E-mail: vrstamenkovic@anl.gov |
|||
|
Web of Science Category: Multidisciplinary Sciences |
|||
|
|
|
|
|
|
|
|
|
|
|
18. Kovtyukhova, N.I., Ollivier, P.J., Martin, B.R., Mallouk, T.E., Chizhik, S.A., Buzaneva, E.V. and Gorchinskiy, A.D. (1999), Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chemistry of Materials, 11 (3), 771-778. |
|||
|
Times Cited in Web of Science Core Collection: 2466 |
|||
|
Abstract: Unilamellar colloids of graphite oxide (GO) were prepared from natural graphite and were grown as monolayer and multilayer thin films on cationic surfaces by electrostatic self-assembly. The multilayer films were grown by alternate adsorption of anionic GO sheets and cationic poly(allylamine hydrochloride) (PAH). The monolayer films consisted of 11-14 Angstrom thick GO sheets, with lateral dimensions between 150 nm and 9 mu m. Silicon substrates primed with amine monolayers gave partial GO monolayers, but surfaces primed with Al13O4-(OH)(24)(H2O)(12)(7+) ions gave densely tiled films that covered approximately 90% of the surface. When alkaline GO colloids were used, the monolayer assembly process selected the largest sheets (from 900 nm to 9 mu m) from the suspension. In this case, many of the flexible sheets appeared folded in AFM images. Multilayer (GO/PAH)(n) films were invariably thicker than expected from the individual thicknesses of the sheets and the polymer monolayers, and this behavior is also attributed to folding of the sheets. Multilayer (GO/PAH), and (GO/polyaniline)(n) films grown between indium-tin oxide and Pt electrodes show diodelike behavior, and higher currents are observed with the conductive polyaniline-containing films. The resisitivity of these films is decreased, as expected, by partial reduction of GO to carbon. |
|||
|
Addresses: Nina I. Kovtyukhova: Institute of Surface Chemistry, National Academy of Sciences of Ukraine, 31, Pr. Nauky, 252022 Kyiv, Ukraine Patricia J. Ollivier: Department of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA Benjamin R. Martin: Department of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA Thomas E. Mallouk: Department of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA Sergey A. Chizhik: Metal-Polymer Research Institute, 32A Kirov Street, Gomel, 246652, Belarus, Ukraine Eugenia V. Buzaneva: National T. Shevchenko University, 64, Vladimirskaya Str., 252033 Kyiv, Ukraine Alexandr D. Gorchinskiy: National T. Shevchenko University, 64, Vladimirskaya Str., 252033 Kyiv, Ukraine |
|||
|
Present addresses: Nina I. Kovtyukhova: Department of Chemistry, The Pennsylvania State University, University Park, Pennsylvania 16802, USA, E-mail: nina@chem.psu.edu |
|||
|
Reprint Address: Nina I. Kovtyukhova: Institute of Surface Chemistry, National Academy of Sciences of Ukraine, 31, Pr. Nauky, 252022 Kyiv, Ukraine |
|||
|
Web of Science Category: Physical Chemistry; Multidisciplinary Materials Science |
|||
|
|
|
|
19. Ho, Y.S. and McKay, G. (1998), Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70 (2), 115-124. |
|
|
Times Cited in Web of Science Core Collection: 2354 |
|
|
Author Keywords: Peat; Lead; Copper; Nickel; Kinetics and Sorption |
|
|
Abstract: A pseudo-second order rate equation describing the kinetics of sorption of divalent metal ions onto sphagnum moss peat at different initial metal ion concentrations and pear doses has been developed. The kinetics of sorption were followed based on the amounts of metal sorbed at various time intervals. Results show that sorption (chemical bonding) might be rate-limiting in the sorption of divalent metal ions onto peat during agitated batch contact time experiments. The rate constant, the equilibrium sorption capacity and the initial sorption rate were calculated. From these parameters, an empirical model for predicting the sorption capacity of metal ions sorbed was derived. (C) 2000 Elsevier Science Ltd. All rights reserved. |
|
|
Addresses: Hong Kong University Science & Technology, Department of Chemical Engineering, Hong Kong, People’s R China |
|
|
Present addresses: Yuh-Shan Ho: Asia University, Water Research Centre, Taichung 41354, Taiwan. E-mail: ysho@asia.edu.tw Gordon McKay: Hamad Bin Khalifa University, Sustainable Development, College of Science & Engineering, E-mail: gmckay@qf.org.qa |
|
|
Reprint Address: McKay, G, Hong Kong University Science & Technology, Department of Chemical Engineering, Hong Kong, People’s R China |
|
|
Web of Science Category: Environmental Engineering; Environmental Sciences; Water Resources |
|
|
|
|
|
|
|
|
|
|
|
|
20. Choi, H.S., Liu, W., Misra, P., Tanaka, E., Zimmer, J.P., Ipe, B.I., Bawendi, M.G. and Frangioni, J.V. (2007), Renal clearance of quantum dots. Nature Biotechnology, 25 (10), 1165-1170. |
|||
|
Times Cited in Web of Science Core Collection: 2345 |
|||
|
Author Keywords: |
|||
|
Abstract: The field of nanotechnology holds great promise for the diagnosis and treatment of human disease. However, the size and charge of most nanoparticles preclude their efficient clearance from the body as intact nanoparticles. Without such clearance or their biodegradation into biologically benign components, toxicity is potentially amplified and radiological imaging is hindered. Using intravenously administered quantum dots in rodents as a model system, we have precisely defined the requirements for renal filtration and urinary excretion of inorganic, metal-containing nanoparticles. Zwitterionic or neutral organic coatings prevented adsorption of serum proteins, which otherwise increased hydrodynamic diameter by > 15 nm and prevented renal excretion. A final hydrodynamic diameter <5.5 nm resulted in rapid and efficient urinary excretion and elimination of quantum dots from the body. This study provides a foundation for the design and development of biologically targeted nanoparticles for biomedical applications. |
|||
|
Addresses: Hak Soo Choi: Division of Hematology/Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Room SL-B05, Boston, Massachusetts 02215, USA. Wenhao Liu: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. Preeti Misra: Division of Hematology/Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Room SL-B05, Boston, Massachusetts 02215, USA. Eiichi Tanaka: Division of Hematology/Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Room SL-B05, Boston, Massachusetts 02215, USA. John P. Zimmer: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. Binil Itty Ipe: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. Moungi G. Bawendi: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. |
|||
|
Present addresses: Hak Soo Choi: Wenhao Liu: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. Preeti Misra: Eiichi Tanaka: John P. Zimmer: Smith Moore Leatherwood LLP, Charlotte, 101 N. Tryon St., Suite 1300 NC, USA. E-mail: john.zimmer@smithmoorelaw.com Binil Itty Ipe: Moungi G. Bawendi: Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail Addresseses: mgb@mit.edu John V. Frangioni: |
|||
|
Reprint Address: John V. Frangioni (reprint author), Beth Israel Deaconess Med Ctr, Dept Med, Div Hematol Oncol, 330 Brookline Ave, Room SL-BO5, Boston, MA 02215 USA. E-mail Addresseses: jfrangio@bidmc.harvard.edu Moungi G. Bawendi (reprint author), Department of Chemistry, Massachusetts Institute of Technology, Building 6-221, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail Addresseses: mgb@mit.edu |
|||
|
Web of Science Category: Biotechnology & Applied Microbiology |
|||
|
|
|
|
21. Kang, B. and Ceder, G. (2009), Battery materials for ultrafast charging and discharging. Nature, 458 (7235), 190-193. |
|
|
Times Cited in Web of Science Core Collection: 2327 |
|
|
Abstract: The storage of electrical energy at high charge and discharge rate is an important technology in today's society, and can enable hybrid and plug-in hybrid electric vehicles and provide back-up for wind and solar energy. It is typically believed that in electrochemical systems very high power rates can only be achieved with supercapacitors, which trade high power for low energy density as they only store energy by surface adsorption reactions of charged species on an electrode material(1-3). Here we show that batteries(4,5) which obtain high energy density by storing charge in the bulk of a material can also achieve ultrahigh discharge rates, comparable to those of supercapacitors. We realize this in LiFePO(4) (ref. 6), a material with high lithium bulk mobility(7,8), by creating a fast ion-conducting surface phase through controlled off-stoichiometry. A rate capability equivalent to full battery discharge in 10-20 s can be achieved. |
|
|
Addresses: Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA |
|
|
Present addresses: Byoungwoo Kang: Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail: Gerbrand Ceder: Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail: gceder@mit.edu |
|
|
Reprint Address: Ceder, Gerbrand (reprint author), Department of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA. E-mail: gceder@mit.edu |
|
|
Web of Science Category: Multidisciplinary Sciences |
|
|
|
|
|
|
22. Decher, G., Hong, J.D. and Schmitt, J. (1992), Buildup of ultrathin multilayer films by a self-assembly process. III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films, 210 (1-2), 831-835. |
||
|
Times Cited in Web of Science Core Collection: 2325 |
||
|
Abstract: Abstract: A solid substrate with a positively charged planar surface is immersed in a solution containing an anionic polyelectrolyte and a monolayer of the polyanion is adsorbed. Since the adsorption is carried out at relatively high concentrations of polyelectrolyte, a large number of ionic residues remain exposed to the interface with the solution and thus the surface charge is effectively reversed. After rinsing in pure water the substrate is immersed in the solution containing a cationic polyelectrolyte. Again a monolayer is adsorbed but now the original surface charge is restored. By repeating both steps in a cyclic fashion, alternating multilayer assemblies of both polymers are obtained. The buildup of the multilayer films was followed by UV/vis spectroscopy and small angle X-ray scattering (SAXS). It is demonstrated that multilayer films composed of at least 100 consecutively alternating layers can be assembled. |
||
|
Addresses: Inslitut fur Physikalische Chemie, Johannes Gutenberg-Universitiit, Welder Weg II, D-6500 Main, Germany |
||
|
Present addresses: Gero Decher: Johannes Gutenberg-Universität Mainz, Mainz, Rhineland-Palatinate, Germany Jong-Dal Hong: Johannes Gutenberg-Universität Mainz, Mainz, Rhineland-Palatinate, Germany Johannes Schmitt: Johannes Gutenberg-Universität Mainz, Mainz, Rhineland-Palatinate, Germany |
||
|
Reprint Address: Decher, Gero (reprint author), Univ Mainz, Inst Phys Chem, W-6500 Mainz, GERMANY |
||
|
Web of Science Category: Multidisciplinary Materials Science; Coatings & Films Materials Science; Applied Physics; Condensed Matter Physics |
||
|
C.H. Giles |
T.H. MacEwan |
S.N. Nakhwa |
D. Smith |
|
23. Giles, C.H., MacEwan, T.H., Nakhwa, S.N. and Smith, D. (1960), Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. Journal of the Chemical Society, 60, 3973-3993. |
|||
|
Times Cited in Web of Science Core Collection: 2292 |
|||
|
Address: Department of Chemical Technology, the Royal College of Science and Technology, Glasgow, UK |
|||
|
Web of Science Category: Multidisciplinary Chemistry |
|||
|
Matthias Thommes |
Katsumi Kaneko |
Alexander V. Neimark |
James P. Olivier |
|
Francisco Rodriguez-Reinoso |
Jean Rouquerol |
Kenneth S. W. Sing |
|
|
24. Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J. and Sing, K.S.W. (2015), Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87 (9-10), 1051-1069. DOI: 10.1515/pac-2014-1117. |
|||
|
Times Cited in Web of Science Core Collection: 2227 |
|||
|
Author Keywords: |
|||
|
Abstract: Gas adsorption is an important tool for the characterisation of porous solids and fine powders. Major advances in recent years have made it necessary to update the 1985 IUPAC manual on Reporting Physisorption Data for Gas/Solid Systems. The aims of the present document are to clarify and standardise the presentation, nomenclature and methodology associated with the application of physisorption for surface area assessment and pore size analysis and to draw attention to remaining problems in the interpretation of physisorption data. |
|||
|
Addresses: Quantachrome Instruments, Dept Appl Sci, Boynton Beach, FL 33426 USA Shinshu Univ, Ctr Energy & Environm Sci, Nagano, Japan Rutgers State Univ, Dept Chem & Biochem Engn, Piscataway, NJ USA Micromerit Instrument Corp, Norcross, NJ USA Univ Alicante, Dept Quim Inorgan, Lab Mat Avanzados, E-03080 Alicante, Spain Aix Marseille Univ, Lab MADIREL, Ctr St Jerome, Marseilles, France Brunel Univ, London, England |
|||
|
Present addresses: Matthias Thommes: Katsumi Kaneko: Alexander V. Neimark: James P. Olivier: Francisco Rodriguez-Reinoso: Jean Rouquerol: Kenneth S. W. Sing: |
|||
|
Reprint Address: Thommes, M (reprint author), Quantachrome Instruments, Dept Appl Sci, 1900 Corp Dr, Boynton Beach, FL 33426 USA. E-mail: matthias.thommes@quantachrome.com |
|||
|
Web of Science Category: Multidisciplinary Chemistry |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25. Nazeeruddin, M.K., Péchy, P., Renouard, T., Zakeeruddin, S.M., Humphry-Baker, R., Comte, P., Liska, P., Cevey, L., Costa, E., Shklover, V., Spiccia, L., Deacon, G.B., Bignozzi, C.A. and Gratzel, M. (2001), Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. Journal of the American Chemical Society, 123 (8), 1613-1624. |
||||
|
Times Cited in Web of Science Core Collection: 2184 |
||||
|
Abstract: A new series of panchromatic ruthenium(II) sensitizers derived from carboxylated terpyridyl complexes of tris-thiocyanato Ru(II) have been developed. Black dye containing different degrees of protonation {(C2H5)(3)NH}[Ru(H(3)tcterpy)(NCS)(3)] 1, {(C4H9)(4)N}(2)[Ru(H(2)tcterpy)(NCS)(3)] 2, {(C4H9)(4)N}(3)[RU(Htcterpy)(NCS)(3)] 3, and {(C4H9)(4)N}(4)[Ru(tcterpy)(NCS)(3)] 4 (tcterpy = 4,4',4 “ -tricarboxy-2,2':6',2 “ -terpyridine) have been synthesized and fully characterized by UV-vis, emission, IR, Raman, NMR, cyclic voltammetry, and X-ray diffraction studies. The crystal structure of complex 2 confirms the presence of a Ru(II)N6 central core derived from the terpyridine ligand and three N-bonded thiocyanates. Intermolecular H-bonding between carboxylates on neighboring terpyridines gives rise to 2-D H-bonded arrays. The absorption and emission maxima of the black dye show a bathochromic shift with decreasing pH and exhibit pH-dependent excited-state lifetimes. The red-shift of the emission maxima is due to better pi -acceptor properties of the acid form that lowers the energy of the CT excited state. The low-energy metal-to-ligand charge-transfer absorption band showed marked solvatochromism due to the presence of thiocyanate ligands. The Ru(II)/(III) oxidation potential of the black dye and the ligand-based reduction potential shifted cathodically with decreasing number of protons and showed more reversible character. The adsorption of complex 3 from methoxyacetonitrile solution onto transparent TiO2 films was interpreted by a Langmuir isotherm yielding an adsorption equilibrium constant, K-ads, of (1.0 +/- 0.3) x 10(5) M-1. The amount of dye adsorbed at monolayer saturation was (n(alpha) = 6.9 +/- 0.3) x 10(-8) mol/mg of TiO2, which is around 30% less than that of the cis-di(thiocyanato)bis(2,2'-bipyridyl-4,4'-dicarboxylate)-ruthenium(II) complex. The black dye, when anchored to nanocrystalline;TiO2. films achieves very efficient sensitization over the whole visible range extending into the near-IR region up to 920 nm, yielding over 80% incident photon to-current efficiencies (IPCE). solar cells containing the black dye were subjected to analysis by a photovoltaic calibration laboratory (NREL, U.S.A.) to determine their solar-to-electric conversion efficiency under standard AM 1.5 sunlight. A short circuit photocurrent density obtained was 20.5 mA/cm(2), and the open circuit voltage was 0.72 V corresponding to an overall conversion efficiency of 10.4%. |
||||
|
Addresses: Contribution from the Laboratory for Photonics and Interfaces, Institute of Physical Chemistry, Swiss Federal Institute of Technology, CH-1015 Lausanne, Switzerland |
||||
|
Present addresses: Md Khaja Nazeeruddin: Contribution from the Laboratory for Photonics and Interfaces, Institute of Physical Chemistry, Swiss Federal Institute of Technology, CH-1015 Lausanne, Switzerland. E-mail: Mdkhaja.Nazeeruddin@epfl.ch Peter Péchy: École Polytechnique Fédérale de Lausanne, Chemistry and Chemical Engineering Section, Switzerland Thierry Renouard: Shaik M. Zakeeruddin: Robin Humphry-Baker: Pascal Comte: Paul Liska: Le Cevey: Emiliana Costa: Dipartimento de Chimica, Università di Ferrara, 44100 Ferrara, Italy Valery Shklover: Laboratory of Crystallography, Swiss Federal Institute of Technology, 8092 Zurich, Switzerland Leone Spiccia: Centre for Green Chemistry and Department of Chemistry, Monash University, Clayton, Vic. 3168, Australia. E-mail: Leone.Spiccia@monash.edu Glen B. Deacon: Centre for Green Chemistry and Department of Chemistry, Monash University, Clayton, Vic. 3168, Australia. E-mail: Glen.Deacon@monash.edu Carlo A. Bignozzi: Dipartimento de Chimica, Università di Ferrara, 44100 Ferrara, Italy Michael Gra1tzel: |
||||
|
Reprint Address: Nazeeruddin, MK (reprint author), Contribution from the Laboratory for Photonics and Interfaces, Institute of Physical Chemistry, Swiss Federal Institute of Technology, CH-1015 Lausanne, Switzerland E-mail Addresses: Mdkhaja.Nazeeruddin@epfl.ch |
||||
|
Web of Science Category: Multidisciplinary Chemistry |
||||
|
|
|
|
|
26. Springer, T.E., Zawodzinski, T.A. and Gottesfeld, S. (1991), Polymer electrolyte fuel cell model. Journal of the Electrochemical Society, 138 (8), 2334-2342. |
||
|
Times Cited in Web of Science Core Collection: 2171 |
||
|
Abstract: We present here an isothermal, one-dimensional, steady-state model for a complete polymer electrolyte fuel cell (PEFC) with a 117 Nafion(R) membrane. In this model we employ water diffusion coefficients electro-osmotic drag coefficients, water sorption isotherms, and membrane conductivities, all measured in our laboratory as functions of membrane water content. The model predicts a net-water-per-proton flux ratio of 0.2 H2O/H+ under typical operating conditions, which is much less than the measured electro-osmotic drag coefficient for a fully hydrated membrane. It also predicts an increase in membrane resistance with increased current density and demonstrates the great advantage of a thinner membrane in alleviating this resistance problem. Both of these predictions were verified experimentally under certain conditions. |
||
|
Present addresses: T.E. Springer: T.A. Zawodzinski: S. Gottesfeld: |
||
|
Reprint Address: Springer, TE, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA |
||
|
Web of Science Category: Electrochemistry; Coatings & Films Materials Science |
||
|
|
|
|
|
|
|
27. Idota, Y., Kubota, T., Matsufuji, A., Maekawa, Y. and Miyasaka, T. (1997), Tin-based amorphous oxide: A high-capacity lithium-ion-storage material. Science, 276 (5317), 1395-1397. |
||||
|
Times Cited in Web of Science Core Collection: 2149 |
||||
|
Abstract: A high-capacity lithium-storage material in metal-oxide form has been synthesized that can replace the carbon-based lithium intercalation materials currently in extensive use as the negative electrode (anode) of lithium-ion rechargeable batteries. This tin-based amorphous composite oxide (TCO) contains Sn(II)-O as the active center for lithium insertion and other glass-forming elements, which make up an oxide network. The TCO anode yields a specific capacity for reversible lithium adsorption more than 50 percent higher than those of the carbon families that persists after charge-discharge cycling when coupled with a lithium cobalt oxide cathode. Lithium-7 nuclear magnetic resonance measurements evidenced the high ionic state of lithium retained in the charged state, in which TCO accepted 8 moles of lithium ions per unit mole. |
||||
|
Addresses: Y. Idota and T. Kubota, Fujifilm Celltec, Matsuzakadaira 1-6, Taiwa-cho, Kurokawa-gun, Miyagi 981-34, Japan A. Matsufuji, Y. Maekawa, T. Miyasaka, Ashigara Re- search Laboratories, Fuji Photo Film, Nakanuma 210, Minamiashigara, Kanagawa 250-01, Japan |
||||
|
Present addresses: Yoshio Idota: Tadahiko Kubota: Akihiro Matsufuji: Yukio Maekawa: Tsutomu Miyasaka: Toin University of Yokohama, Graduate School of Engineering, Japan. E-mail: miyasaka@toin.ac.jp |
||||
|
Web of Science Category: Multidisciplinary Sciences |
||||
|
|
|
|
28. Henkelman, G. and Jónsson, H. (2000), Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. Journal of Chemical Physics, 113 (22), 9978-9985. |
|
|
Times Cited in Web of Science Core Collection: 2136 |
|
|
Abstract: An improved way of estimating the local tangent in the nudged elastic band method for finding minimum energy paths is presented. In systems where the force along the minimum energy path is large compared to the restoring force perpendicular to the path and when many images of the system are included in the elastic band, kinks can develop and prevent the band from converging to the minimum energy path. We show how the kinks arise and present an improved way of estimating the local tangent which solves the problem. The task of finding an accurate energy and configuration for the saddle point is also discussed and examples given where a complementary method, the dimer method, is used to efficiently converge to the saddle point. Both methods only require the first derivative of the energy and can, therefore, easily be applied in plane wave based density-functional theory calculations. Examples are given from studies of the exchange diffusion mechanism in a Si crystal, Al addimer formation on the Al(100) surface, and dissociative adsorption of CH4 on an Ir(111) surface. (C) 2000 American Institute of Physics. [S0021-9606(00)70546-0]. |
|
|
Addresses: Department of Chemistry, Box 351700, University of Washington, Seattle, Washington 98195-1700 USA |
|
|
Present addresses: Graeme Henkelman: University of Texas Austin, Department of Chemistry & Biochemistry, Austin, TX 78712 USA. E-mail: henkelman@mail.utexas.edu Hannes Jónsson: University of Iceland, Chemistry division of the Science Institute, Iceland; E-mail: hj@hi.is |
|
|
Reprint Address: Henkelman, G (reprint author), Univ Washington, Dept Chem, Box 351700, Seattle, WA 98195 USA |
|
|
Web of Science Category: Molecular & Chemical Atomic Physics |
|
|
|
|
|
|
|
|
|
29. Chmiola, J., Yushin, G., Gogotsi, Y., Portet, C., Simon, P. and Taberna, P.L. (2006), Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science, 313 (5794), 1760-1763. |
|||||
|
Times Cited in Web of Science Core Collection: 2124 |
|||||
|
Abstract: Carbon supercapacitors, which are energy storage devices that use ion adsorption on the surface of highly porous materials to store charge, have numerous advantages over other power-source technologies, but could realize further gains if their electrodes were properly optimized. Studying the effect of the pore size on capacitance could potentially improve performance by maximizing the electrode surface area accessible to electrolyte ions, but until recently, no studies had addressed the lower size limit of accessible pores. Using carbide-derived carbon, we generated pores with average sizes from 0.6 to 2.25 nanometer and studied double-layer capacitance in an organic electrolyte. The results challenge the long-held axiom that pores smaller than the size of solvated electrolyte ions are incapable of contributing to charge storage. |
|||||
|
Addresses: Department of Materials Science and Engineering and A. J. Drexel Nanotechnology Institute, Drexel University, Philadelphia, PA 19104, USA. Universite´ Paul Sabatier, CIRIMAT, UMR CNRS 5085, 31062 Toulouse Cedex 4, France. |
|||||
|
Present addresses: John Chmiola: Gleb Yushin: Georgia Institute of Technology, School of Materials Science and Engineering, Atlanta, USA. E-mail: yushin@gatech.edu Yury Gogotsi: Drexel University, Department of Materials Science and Engineering, Philadelphia, USA. E-mail: gogotsi@drexel.edu Cristelle Portet: P. Simon: Paul Sabatier University - Toulouse III, Centre Inter-universitaire de Recherche et d'Ingénierie en Matériaux (CIRIMAT), France P. L. Taberna: European Space Agency, Lutetia Parisorum, Île-de-France, France |
|||||
|
Reprint Address: Gogotsi, Y (reprint author), Drexel Univ, Dept Mat Sci & Engn, Philadelphia, PA 19104 USA. E-mail Addresseses: gogotsi@drexel.edu |
|||||
|
Web of Science Category: Multidisciplinary Sciences |
|||||
|
|
|
|
30. Myers, A.L. and Prausnitz, J.M. (1965), Thermodynamics of mixed-gas adsorption. AIChE Journal, 11 (1), 121-127. |
|
|
Times Cited in Web of Science Core Collection: 2119 |
|
|
Abstract: A simple technique is described for calculating the adsorption equilibria for components in a gaseous mixture, using only data for the pure-component adsorption equilibria at the same temperature and on the same adsorbent. The proposed technique is based on the concept of an ideal adsorbed solution and, using classical surface thermodynamics, an expression analogous to Raoult’s law is obtained. The essential idea of the calculation lies in the recognition that in an ideal solution the partial pressure of an adsorbed component is given by the product of its mole fraction in the adsorbed phase and the pressure which it would exert as a pure adsorbed Component at the same temperature and spreading pressure as those of the mixture. Predicted isotherms give excellent agreement with experimental data for methaneethane and ethylene-carbon dioxide on activated carbon and for carbon monoxide-oxygen and propane-propylene on silica gel. The simDlicitv of the calculation, which requires no data for the mixture, makes it especially useful for engineering applications. |
|
|
Present addresses: Myers, A.L.: John M. Prausnitz: University of California, Berkeley, Chemical and Biomolecular Engineering, California, USA, E-mail: Prausnit@cchem.berkeley.edu |
|
|
Reprint Address: |
|
|
Web of Science Category: Chemical Engineering |
|
|
|
|
31. Boyden, S.V. (1951), The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. Journal of Experimental Medicine, 93 (2), 107-120. |
|
Times Cited in Web of Science Core Collection: 2087 |
|
Addresses: Laboratories of the Rockefeller Institute for Medical Research; Animal Health Trust, London, England. |
|
Web of Science Category: Immunology; Research & Experimental Medicine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32. Furukawa, H., Ko, N., Go, Y.B., Aratani, N., Choi, S.B., Choi, E., Yazaydin, A.O., Snurr, R.Q., O’Keeffe, M., Kim, J. and Yaghi, O.M. (2010), Ultrahigh porosity in metal-organic frameworks. Science, 329 (5990), 424-428. |
|||||
|
Times Cited in Web of Science Core Collection: 2041 |
|||||
|
Abstract: Crystalline solids with extended non-interpenetrating three-dimensional crystal structures were synthesized that support well-defined pores with internal diameters of up to 48 angstroms. The Zn(4)O(CO(2))(6) unit was joined with either one or two kinds of organic link, 4,4’,4”-[benzene-1,3,5-triyl-tris (ethyne-2,1-diyl)]tribenzoate (BTE), 4,4’,44”-[benzene-1,3,5-triyl-tris(benzene-4,1-diyl)]tribenzoate (BBC), 4,4’,44”-benzene-1,3,5-triyl-tribenzoate (BTB)/2,6-naphthalenedicarboxylate (NDC), and BTE/biphenyl-4,4’-dicarboxylate (BPDC), to give four metal-organic frameworks (MOFs), MOF-180, -200, -205, and -210, respectively. Members of this series of MOFs show exceptional porosities and gas (hydrogen, methane, and carbon dioxide) uptake capacities. For example, MOF-210 has Brunauer-Emmett-Teller and Langmuir surface areas of 6240 and 10,400 square meters per gram, respectively, and a total carbon dioxide storage capacity of 2870 milligrams per gram. The volume-specific internal surface area of MOF-210 (2060 square meters per cubic centimeter) is equivalent to the outer surface of nanoparticles (3-nanometer cubes) and near the ultimate adsorption limit for solid materials. |
|||||
|
Addresses: Nakeun Ko: Soongsil Univ, Dept Chem, Seoul 156743, South Korea Sang Beom Choi: Soongsil Univ, Dept Chem, Seoul 156743, South Korea Jaheon Kim: Soongsil Univ, Dept Chem, Seoul 156743, South Korea Hiroyasu Furukawa: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Yong Bok Go: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Naoki Aratani: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Eunwoo Choi: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Michael O’Keeffe: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Omar M. Yaghi: Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA Hiroyasu Furukawa: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. Yong Bok Go: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. Naoki Aratani: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. Eunwoo Choi: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. Michael O’Keeffe: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. Omar M. Yaghi: Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA. A. Özgür Yazaydin: Northwestern Univ, Dept Chem & Biol Engn, Evanston, IL 60208 USA. Randall Q. Snurr: Northwestern Univ, Dept Chem & Biol Engn, Evanston, IL 60208 USA. Omar M. Yaghi: Univ Calif Los Angeles, Inst Genom & Prote, Dept Energy, Los Angeles, CA 90095 USA. |
|||||
|
Present addresses: Hiroyasu Furukawa: Department of Chemistry, University of California, 636 Latimer Hall, Berkeley, CA 94720, USA. E-mail: furukawa@berkeley.edu Nakeun Ko: Department of Chemistry, Soongsil University, Seoul, South Korea. Yong Bok Go: Rutgers, The State University of New Jersey, New Brunswick, New Jersey, USA Naoki Aratani: University of California, Los Angeles, Los Angeles, California, USA. Sang Beom Choi: Soongsil University, Sŏul, Seoul, South Korea. Eunwoo Choi: Northwestern University, Evanston, IL, USA. A. Özgür Yazaydin: Northwestern University, Evanston, Illinois, USA. Randall Q. Snurr: Northwestern University, Evanston, Illinois, USA. Michael O’Keeffe: Arizona State University, Phoenix, Arizona, USA. Jaheon Kim: Department of Chemistry, Soongsil University, Seoul, South Korea. Omar M. Yaghi: University of California, Berkeley, Berkeley, California, USA. E-mail: yaghi@berkeley.edu |
|||||
|
Reprint Address: Kim, J (reprint author), Soongsil Univ, Dept Chem, Seoul 156743, South Korea. E-mail: jaheon@ssu.ac.kr; yaghi@chem.ucla.edu |
|||||
|
Web of Science Category: Multidisciplinary Sciences |
|||||
|
|
|
|
|
33. Karickhoff, S.W., Brown, D.S. and Scott, T.A. (1979), Sorption of hydrophobic pollutants on natural sediments. Water Research, 13 (3), 241-248. |
||
|
Times Cited in Web of Science Core Collection: 2004 |
||
|
Address: Environmental Research Laboratory, U.S. Environmental Protection Agency, College Station Road, Athens, GA 30605, U.S.A. |
||
|
Reprint Address: Karickhoff, SW, U.S. Environmental Protection Agency, Environmental Research Laboratory, College Station Road, Athens, GA 30605, USA |
||
|
Present addresses: S.W. Karickhoff, D.S. Brown, T.A. Scott, |
||
|
Web of Science Category: Environmental Engineering; Environmental Sciences; Water Resources |
||
Title: Adsorption of gases in multimolecular layers
Author(s): Brunauer, S (Brunauer, S); Emmett, PH (Emmett, PH); Teller, E (Teller, E)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 60 Pages: 309-319 DOI: 10.1021/ja01269a023 Published: JAN-JUN 1938
Times Cited in Web of Science Core Collection: 17788
Total Times Cited: 18045
Document Type: Article
Addresses: George Washington Univ, Bur Chem & Soils, Washington, DC USA.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: ORDERED MESOPOROUS MOLECULAR-SIEVES SYNTHESIZED BY A LIQUID-CRYSTAL TEMPLATE MECHANISM
Author(s): KRESGE, CT (KRESGE, CT); LEONOWICZ, ME (LEONOWICZ, ME); ROTH, WJ (ROTH, WJ); VARTULI, JC (VARTULI, JC); BECK, JS (BECK, JS)
Source: NATURE Volume: 359 Issue: 6397 Pages: 710-712 DOI: 10.1038/359710a0 Published: OCT 22 1992
Times Cited in Web of Science Core Collection: 13637
Total Times Cited: 14360
Abstract: MICROPOROUS and mesoporous inorganic solids (with pore diameters of less-than-or-equal-to 20 angstrom and approximately 20-500 angstrom respectively)1 have found great utility as catalysts and sorption media because of their large internal surface area. Typical microporous materials are the crystalline framework solids, such as zeolites2, but the largest pore dimensions found so far are approximately 10-12 angstrom for some metallophosphates3-5 and approximately 14 angstrom for the mineral cacoxenite6. Examples of mesoporous solids include silicas7 and modified layered materials8-11, but these are invariably amorphous or paracrystalline, with pores that are irregularly spaced and broadly distributed in size8,12. Pore size can be controlled by intercalation of layered silicates with a surfactant species9,13, but the final product retains, in part, the layered nature of the precursor material. Here we report the synthesis of mesoporous solids from the calcination of aluminosilicate gels in the presence of surfactants. The material14,15 possesses regular arrays of uniform channels, the dimensions of which can be tailored (in the range 16 angstrom to 100 angstrom or more) through the choice of surfactant, auxiliary chemicals and reaction conditions. We propose that the formation of these materials takes place by means of a liquid-crystal 'templating' mechanism, in which the silicate material forms inorganic walls between ordered surfactant micelles.
Document Type: Article
Addresses: MOBIL RES & DEV CORP,CENT RES LAB,PRINCETON,NJ 08540.
Reprint Address: KRESGE, CT (reprint author), MOBIL RES & DEV CORP,PAULSBORO RES LAB,PAULSBORO,NJ 08066, USA.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: THE ADSORPTION OF GASES ON PLANE SURFACES OF GLASS, MICA AND PLATINUM.
Author(s): Langmuir, I (Langmuir, Irving)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 40 Pages: 1361-1403 DOI: 10.1021/ja02242a004 Published: JUL-DEC 1918
Times Cited in Web of Science Core Collection: 11080
Total Times Cited: 11391
Document Type: Article
Addresses: Gen Elect Co, Res Lab, Schenectady, NY USA.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu
Author(s): Grimme, S (Grimme, Stefan); Antony, J (Antony, Jens); Ehrlich, S (Ehrlich, Stephan); Krieg, H (Krieg, Helge)
Source: JOURNAL OF CHEMICAL PHYSICS Volume: 132 Issue: 15 Article Number: 154104 DOI: 10.1063/1.3382344 Published: APR 21 2010
Times Cited in Web of Science Core Collection: 11034
Total Times Cited: 11063
Abstract: The method of dispersion correction as an add-on to standard Kohn-Sham density functional theory (DFT-D) has been refined regarding higher accuracy, broader range of applicability, and less empiricism. The main new ingredients are atom-pairwise specific dispersion coefficients and cutoff radii that are both computed from first principles. The coefficients for new eighth-order dispersion terms are computed using established recursion relations. System (geometry) dependent information is used for the first time in a DFT-D type approach by employing the new concept of fractional coordination numbers (CN). They are used to interpolate between dispersion coefficients of atoms in different chemical environments. The method only requires adjustment of two global parameters for each density functional, is asymptotically exact for a gas of weakly interacting neutral atoms, and easily allows the computation of atomic forces. Three-body nonadditivity terms are considered. The method has been assessed on standard benchmark sets for inter- and intramolecular noncovalent interactions with a particular emphasis on a consistent description of light and heavy element systems. The mean absolute deviations for the S22 benchmark set of noncovalent interactions for 11 standard density functionals decrease by 15%-40% compared to the previous (already accurate) DFT-D version. Spectacular improvements are found for a tripeptide-folding model and all tested metallic systems. The rectification of the long-range behavior and the use of more accurate C-6 coefficients also lead to a much better description of large (infinite) systems as shown for graphene sheets and the adsorption of benzene on an Ag(111) surface. For graphene it is found that the inclusion of three-body terms substantially (by about 10%) weakens the interlayer binding. We propose the revised DFT-D method as a general tool for the computation of the dispersion energy in molecules and solids of any kind with DFT and related (low-cost) electronic structure methods for large systems.
Document Type: Article
Addresses: [Grimme, Stefan; Antony, Jens; Ehrlich, Stephan; Krieg, Helge] Univ Munster, Inst Organ Chem, D-48149 Munster, Germany.
Reprint Address: Grimme, S (reprint author), Univ Munster, Inst Organ Chem, Corrensstr 40, D-48149 Munster, Germany.
E-mail Addresses: grimmes@uni-muenster.de
Web of Science Categories: Chemistry, Physical; Physics, Atomic, Molecular & Chemical
ISSN: 0021-9606
eISSN: 1089-7690
Title: A NEW FAMILY OF MESOPOROUS MOLECULAR-SIEVES PREPARED WITH LIQUID-CRYSTAL TEMPLATES
Author(s): BECK, JS (BECK, JS); VARTULI, JC (VARTULI, JC); ROTH, WJ (ROTH, WJ); LEONOWICZ, ME (LEONOWICZ, ME); KRESGE, CT (KRESGE, CT); SCHMITT, KD (SCHMITT, KD); CHU, CTW (CHU, CTW); OLSON, DH (OLSON, DH); SHEPPARD, EW (SHEPPARD, EW); MCCULLEN, SB (MCCULLEN, SB); HIGGINS, JB (HIGGINS, JB); SCHLENKER, JL (SCHLENKER, JL)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 114 Issue: 27 Pages: 10834-10843 DOI: 10.1021/ja00053a020 Published: DEC 30 1992
Times Cited in Web of Science Core Collection: 9381
Total Times Cited: 9914
Abstract: The synthesis, characterization, and proposed mechanism of formation of a new family of silicate/aluminosilicate mesoporous molecular sieves designated as M41S is described. MCM-41, one member of this family, exhibits a hexagonal arrangement of uniform mesopores whose dimensions may be engineered in the range of approximately 15 angstrom to greater than 100 angstrom. Other members of this family, including a material exhibiting cubic symmetry, have been synthesized. The larger pore M41S materials typically have surface areas above 700 m2/g and hydrocarbon sorption capacities of 0.7 cc/g and greater. A templating mechanism (liquid crystal templating-LCT) in which surfactant liquid crystal structures serve as organic templates is proposed for the formation of these materials. In support of this templating mechanism, it was demonstrated that the structure and pore dimensions of MCM-41 materials are intimately linked to the properties of the surfactant, including surfactant chain length and solution chemistry. The presence of variable pore size MCM-41, cubic material, and other phases indicates that M41S is an extensive family of materials.
Document Type: Article
Addresses: MOBIL RES & DEV CORP, PAULSBORO RES LAB, PAULSBORO, NJ 08066 USA.
Reprint Address: BECK, JS (reprint author), MOBIL RES & DEV CORP, CENT RES LAB, PRINCETON, NJ 08543 USA.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Pseudo-second order model for sorption processes
Author(s): Ho, YS (Ho, YS); McKay, G (McKay, G)
Source: PROCESS BIOCHEMISTRY Volume: 34 Issue: 5 Pages: 451-465 DOI: 10.1016/S0032-9592(98)00112-5 Published: JUL 1999
Times Cited in Web of Science Core Collection: 8169
Total Times Cited: 8612
Abstract: A literature review of the use of sorbents and biosorbents to treat polluted aqueous effluents containing dyes/organics or metal ions has been conducted. Over 70 systems have been reported since 1984 and over 43 of these reported the mechanism as being a pseudo-first order kinetic mechanism. Three sorption kinetic models are presented in this paper and have been used to test 11 of the literature systems previously reported as first order kinetics and one system previously reported as a second order process. In all 12 systems, the highest correlation coefficients were obtained for the pseudo-second order kinetic model. (C) 1999 Elsevier Science Ireland Ltd. All rights reserved.
Document Type: Article
Addresses: Hong Kong Univ Sci & Technol, Dept Chem Engn, Hong Kong, Peoples R China.
Reprint Address: McKay, G (reprint author), Hong Kong Univ Sci & Technol, Dept Chem Engn, Hong Kong, Peoples R China.
Web of Science Categories: Biochemistry & Molecular Biology; Biotechnology & Applied Microbiology; Engineering, Chemical
ISSN: 1359-5113
eISSN: 1873-3298
Title: A climbing image nudged elastic band method for finding saddle points and minimum energy paths
Author(s): Henkelman, G (Henkelman, G); Uberuaga, BP (Uberuaga, BP); Jonsson, H (Jonsson, H)
Source: JOURNAL OF CHEMICAL PHYSICS Volume: 113 Issue: 22 Pages: 9901-9904 Article Number: PII [S0021-9606(00)71246-3] DOI: 10.1063/1.1329672 Published: DEC 8 2000
Times Cited in Web of Science Core Collection: 7412
Total Times Cited: 7450
Abstract: A modification of the nudged elastic band method for finding minimum energy paths is presented. One of the images is made to climb up along the elastic band to converge rigorously on the highest saddle point. Also, variable spring constants are used to increase the density of images near the top of the energy barrier to get an improved estimate of the reaction coordinate near the saddle point. Applications to CH4 dissociative adsorption on Ir(111) and H-2 on Si(100) using plane wave based density functional theory are presented. (C) 2000 American Institute of Physics. [S0021-9606(00)71246-3].
Document Type: Article
Addresses: Univ Washington, Dept Chem 351700, Seattle, WA 98195 USA.
Univ Washington, Dept Phys 351560, Seattle, WA 98195 USA.
Reprint Address: Henkelman, G (reprint author), Univ Washington, Dept Chem 351700, Seattle, WA 98195 USA.
Web of Science Categories: Chemistry, Physical; Physics, Atomic, Molecular & Chemical
ISSN: 0021-9606
eISSN: 1089-7690
Title: Nanowire dye-sensitized solar cells
Author(s): Law, M (Law, M); Greene, LE (Greene, LE); Johnson, JC (Johnson, JC); Saykally, R (Saykally, R); Yang, PD (Yang, PD)
Source: NATURE MATERIALS Volume: 4 Issue: 6 Pages: 455-459 DOI: 10.1038/nmat1387 Published: JUN 2005
Times Cited in Web of Science Core Collection: 4676
Total Times Cited: 4756
Abstract: Excitonic solar cells(1)-including organic, hybrid organic inorganic and dye-sensitized cells (DSCs)-are promising devices for inexpensive, large-scale solar energy conversion. The DSC is currently the most efficient(2) and stable(3) excitonic photocell. Central to this device is a thick nanoparticle film that provides a large surface area for the adsorption of light-harvesting molecules. However, nanoparticle DSCs rely on trap-limited diffusion for electron transport, a slow mechanism that can limit device efficiency, especially at longer wavelengths. Here we introduce a version of the dye-sensitized cell in which the traditional nanoparticle film is replaced by a dense array of oriented, crystalline ZnO nanowires. The nanowire anode is synthesized by mild aqueous chemistry and features a surface area up to one-fifth as large as a nanoparticle cell. The direct electrical pathways provided by the nanowires ensure the rapid collection of carriers generated throughout the device, and a full Sun efficiency of 1.5% is demonstrated, limited primarily by the surface area of the nanowire array.
Document Type: Article
Addresses: Univ Calif Berkeley, Dept Chem, Berkeley, CA 94720 USA.
Univ Calif Berkeley, Lawrence Berkeley Lab, Div Mat Sci, Berkeley, CA 94720 USA.
Reprint Address: Law, M (reprint author), Univ Calif Berkeley, Dept Chem, Berkeley, CA 94720 USA.
E-mail Addresses: p_yang@berkeley.edu
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1476-1122
eISSN: 1476-4660
Title: Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals
Author(s): Hammer, B (Hammer, B); Hansen, LB (Hansen, LB); Norskov, JK (Norskov, JK)
Source: PHYSICAL REVIEW B Volume: 59 Issue: 11 Pages: 7413-7421 DOI: 10.1103/PhysRevB.59.7413 Published: MAR 15 1999
Times Cited in Web of Science Core Collection: 3741
Total Times Cited: 3821
Abstract: A simple formulation of a generalized gradient approximation for the exchange and correlation energy of electrons has been proposed by Perdew, Burke, and Ernzerhof (PBE) [Phys. Rev. Lett. 77, 3865 (1996)]. Subsequently Zhang and Yang [Phys. Rev. Lett. 80, 890 (1998)] have shown that a slight revision of the PBE functional systematically improves the atomization energies for a large database of small molecules. In the present work, we show that the Zhang and Yang functional (revPBE) also improves the chemisorption energetics of atoms and molecules on transition-metal surfaces. Our test systems comprise atomic and molecular adsorption of oxygen, CO, and NO on Ni(100), Ni(111), Rh(100), Pd(100), and Pd(111) surfaces. As the revPBE functional may locally violate the Lieb-Oxford criterion, we further develop an alternative revision of the PBE functional, RPBE, which gives the same improvement of the chemisorption energies as the revPBE functional at the same time as it fulfills the Lieb-Oxford criterion locally. [S0163-1829(99)02711-3].
Document Type: Article
Addresses: Univ Aalborg, Inst Phys, DK-9220 Aalborg, Denmark.
Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Phys, DK-2800 Lyngby, Denmark.
Reprint Address: Hammer, B (reprint author), Univ Aalborg, Inst Phys, Pontoppidanstr 103, DK-9220 Aalborg, Denmark.
Web of Science Categories: Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1098-0121
eISSN: 1550-235X
Record 12 of 140
Title: ADSORPTION AND SURFACE-ENHANCED RAMAN OF DYES ON SILVER AND GOLD SOLS
Author(s): LEE, PC (LEE, PC); MEISEL, D (MEISEL, D)
Source: JOURNAL OF PHYSICAL CHEMISTRY Volume: 86 Issue: 17 Pages: 3391-3395 DOI: 10.1021/j100214a025 Published: 1982
Times Cited in Web of Science Core Collection: 3596
Total Times Cited: 3699
Document Type: Article
Addresses: ARGONNE NATL LAB,DIV CHEM,ARGONNE,IL 60439.
Web of Science Categories: Chemistry, Physical
ISSN: 0022-3654
Title: Size- and support-dependency in the catalysis of gold
Author(s): Haruta, M (Haruta, M)
Source: CATALYSIS TODAY Volume: 36 Issue: 1 Pages: 153-166 DOI: 10.1016/S0920-5861(96)00208-8 Published: APR 25 1997
Times Cited in Web of Science Core Collection: 3355
Total Times Cited: 3404
Abstract: The adsorption properties and reactivities of gold are summarized in terms of their size dependency from bulk to fine particles, clusters and atoms. The catalytic performances of gold markedly depend on dispersion, supports, and preparation methods. When gold is deposited on select metal oxides as hemispherical ultra-fine particles with diameters smaller than 5 nn, it exhibits surprisingly high activities and/or selectivities in the combustion of CO and saturated hydrocarbons, the oxidation-decomposition of amines and organic halogenated compounds, the partial oxidation of hydrocarbons, the hydrogenation of carbon oxides, unsaturated carbonyl compounds, alkynes and alkadienes, and the reduction of nitrogen oxides. The unique catalytic nature of supported gold can be explained by assuming that the gold-metal oxide perimeter interface acts as a site for activating at least one of the reactants, for example, oxygen. Some examples and future prospects in applications are also briefly described.
Document Type: Article; Proceedings Paper
Conference Title: Workshop on Environmental Catalysis - The Role of IB Metals
Conference Date: NOV 02-03, 1995
Conference Location: OSAKA NATL RES INST, IKEDA, JAPAN
Conference Host: OSAKA NATL RES INST
Reprint Address: Haruta, M (reprint author), OSAKA NATL RES INST, AIST, MIDORIGAOKA 1-8-31, IKEDA, OSAKA 563, JAPAN.
Web of Science Categories: Chemistry, Applied; Chemistry, Physical; Engineering, Chemical
ISSN: 0920-5861
Title: On a theory of the van der Waals adsorption of gases
Author(s): Brunauer, S (Brunauer, S); Deming, LS (Deming, LS); Deming, WE (Deming, WE); Teller, E (Teller, E)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 62 Pages: 1723-1732 DOI: 10.1021/ja01864a025 Published: JUL-DEC 1940
Times Cited in Web of Science Core Collection: 3309
Total Times Cited: 3422
Document Type: Article
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Storage of hydrogen in single-walled carbon nanotubes
Author(s): Dillon, AC (Dillon, AC); Jones, KM (Jones, KM); Bekkedahl, TA (Bekkedahl, TA); Kiang, CH (Kiang, CH); Bethune, DS (Bethune, DS); Heben, MJ (Heben, MJ)
Source: NATURE Volume: 386 Issue: 6623 Pages: 377-379 DOI: 10.1038/386377a0 Published: MAR 27 1997
Times Cited in Web of Science Core Collection: 3228
Total Times Cited: 3384
Abstract: Pores of molecular dimensions can adsorb large quantities of gases owing to the enhanced density of the adsorbed material inside the pores(1), a consequence of the attractive potential of the pore walls, Pederson and Broughton have suggested(2) that carbon nanotubes, which have diameters of typically a few nanometres, should be able to draw up liquids by capillarity, and this effect has been seen for low-surface-tension liquids in large-diameter, multi-walled nanotubes(3). Here we show that a gas can condense to high density inside narrow, single-walled nanotubes (SWNTs), Temperature-programmed desorption spectrosocopy shows that hydrogen will condense inside SWNTs under conditions that do not induce adsorption within a standard mesoporous activated carbon, The very high hydrogen uptake in these materials suggests that they might be effective as a hydrogen-storage material for fuel-cell electric vehicles.
Document Type: Article
Addresses: NATL RENEWABLE ENERGY LAB,GOLDEN,CO 80401.
IBM CORP,ALMADEN RES CTR,DIV RES,SAN JOSE,CA 95120.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries
Author(s): Ji, XL (Ji, Xiulei); Lee, KT (Lee, Kyu Tae); Nazar, LF (Nazar, Linda F.)
Source: NATURE MATERIALS Volume: 8 Issue: 6 Pages: 500-506 DOI: 10.1038/NMAT2460 Published: JUN 2009
Times Cited in Web of Science Core Collection: 2996
Total Times Cited: 3081
Abstract: The Li-S battery has been under intense scrutiny for over two decades, as it offers the possibility of high gravimetric capacities and theoretical energy densities ranging up to a factor of five beyond conventional Li-ion systems. Herein, we report the feasibility to approach such capacities by creating highly ordered interwoven composites. The conductive mesoporous carbon framework precisely constrains sulphur nanofiller growth within its channels and generates essential electrical contact to the insulating sulphur. The structure provides access to Li(+) ingress/egress for reactivity with the sulphur, and we speculate that the kinetic inhibition to diffusion within the framework and the sorption properties of the carbon aid in trapping the polysulphides formed during redox. Polymer modification of the carbon surface further provides a chemical gradient that retards diffusion of these large anions out of the electrode, thus facilitating more complete reaction. Reversible capacities up to 1,320m Ah g(-1) are attained. The assembly process is simple and broadly applicable, conceptually providing new opportunities for materials scientists for tailored design that can be extended to many different electrode materials.
Document Type: Article
Addresses: [Ji, Xiulei; Lee, Kyu Tae; Nazar, Linda F.] Univ Waterloo, Dept Chem, Waterloo, ON N2L 3G1, Canada.
Reprint Address: Ji, XL (reprint author), Univ Waterloo, Dept Chem, Waterloo, ON N2L 3G1, Canada.
E-mail Addresses: lfnazar@uwaterloo.ca
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1476-1122
Title: Exceptional chemical and thermal stability of zeolitic imidazolate frameworks
Author(s): Park, KS (Park, Kyo Sung); Ni, Z (Ni, Zheng); Cote, AP (Cote, Adrien P.); Choi, JY (Choi, Jae Yong); Huang, RD (Huang, Rudan); Uribe-Romo, FJ (Uribe-Romo, Fernando J.); Chae, HK (Chae, Hee K.); O'Keeffe, M (O'Keeffe, Michael); Yaghi, OM (Yaghi, Omar M.)
Source: PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA Volume: 103 Issue: 27 Pages: 10186-10191 DOI: 10.1073/pnas.0602439103 Published: JUL 5 2006
Times Cited in Web of Science Core Collection: 2896
Total Times Cited: 2935
Abstract: Twelve zeolitic imidazolate frameworks (ZIFs; termed ZIF-1 to -12) have been synthesized as crystals by copolymerization of either Zn(II) (ZIF-1 to -4, -6 to -8, and -10 to -11) or Co(II) (ZIF-9 and -12) with imidazolate-type links. The ZIF crystal structures are based on the nets of seven distinct aluminosilicate zeolites: tetrahedral Si(AI) and the bridging O are replaced with transition metal ion and imidazolate link, respectively. In addition, one example of mixed-coordination imidazolate of Zn(II) and In(III) (ZIF-5) based on the garnet net is reported. Study of the gas adsorption and thermal and chemical stability of two prototypical members, ZIF-8 and -11, demonstrated their permanent porosity (Langmuir surface area = 1,810 m(2)/g), high thermal stability (up to 550 degrees C), and remarkable chemical resistance to boiling alkaline water and organic solvents.
Document Type: Article
Addresses: Univ Calif Los Angeles, Dept Chem & Biochem, Ctr Reticular Mat Res, Calif Nanosyst Inst, Los Angeles, CA 90095 USA.
Seoul Natl Univ, Dept Chem Educ, Seoul 151748, South Korea.
Beijing Inst Technol, Sch Sci, Inst Phys Chem, Beijing 100081, Peoples R China.
Arizona State Univ, Dept Chem, Tempe, AZ 85287 USA.
Reprint Address: Yaghi, OM (reprint author), Univ Calif Los Angeles, Dept Chem & Biochem, Ctr Reticular Mat Res, Calif Nanosyst Inst, Los Angeles, CA 90095 USA.
E-mail Addresses: yaghi@chem.ucla.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0027-8424
Title: Origin of the overpotential for oxygen reduction at a fuel-cell cathode
Author(s): Norskov, JK (Norskov, JK); Rossmeisl, J (Rossmeisl, J); Logadottir, A (Logadottir, A); Lindqvist, L (Lindqvist, L); Kitchin, JR (Kitchin, JR); Bligaard, T (Bligaard, T); Jonsson, H (Jonsson, H)
Source: JOURNAL OF PHYSICAL CHEMISTRY B Volume: 108 Issue: 46 Pages: 17886-17892 DOI: 10.1021/jp047349j Published: NOV 18 2004
Times Cited in Web of Science Core Collection: 2802
Total Times Cited: 2815
Abstract: We present a method for calculating the stability of reaction intermediates of electrochemical processes on the basis of electronic structure calculations. We used that method in combination with detailed density functional calculations to develop a detailed description of the free-energy landscape of the electrochemical oxygen reduction reaction over Pt(111) as a function of applied bias. This allowed us to identify the origin of the overpotential found for this reaction. Adsorbed oxygen and hydroxyl are found to be very stable intermediates at potentials close to equilibrium, and the calculated rate constant for the activated proton/electron transfer to adsorbed oxygen or hydroxyl can account quantitatively for the observed kinetics. On the basis of a database of calculated oxygen and hydroxyl adsorption energies, the trends in the oxygen reduction rate for a large number of different transition and noble metals can be accounted for. Alternative reaction mechanisms involving proton/electron transfer to adsorbed molecular oxygen were also considered, and this peroxide mechanism was found to dominate for the most noble metals. The model suggests ways to improve the electrocatalytic properties of fuel-cell cathodes.
Document Type: Article
Addresses: Tech Univ Denmark, Ctr Atom Scale Mat Phys, Dept Phys, DK-2800 Lyngby, Denmark.
Univ Delaware, Dept Chem Engn, Newark, DE 19716 USA.
Univ Iceland, Inst Sci, IS-107 Reykjavik, Iceland.
Univ Iceland, Fac Sci, IS-107 Reykjavik, Iceland.
Reprint Address: Norskov, JK (reprint author), Tech Univ Denmark, Ctr Atom Scale Mat Phys, Dept Phys, DK-2800 Lyngby, Denmark.
E-mail Addresses: norskov@fysik.dtu.dk
Web of Science Categories: Chemistry, Physical
ISSN: 1520-6106
Title: Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability
Author(s): Stamenkovic, VR (Stamenkovic, Vojislav R.); Fowler, B (Fowler, Ben); Mun, BS (Mun, Bongjin Simon); Wang, GF (Wang, Guofeng); Ross, PN (Ross, Philip N.); Lucas, CA (Lucas, Christopher A.); Markovic, NM (Markovic, Nenad M.)
Source: SCIENCE Volume: 315 Issue: 5811 Pages: 493-497 DOI: 10.1126/science.1135941 Published: JAN 26 2007
Times Cited in Web of Science Core Collection: 2595
Total Times Cited: 2631
Abstract: The slow rate of the oxygen reduction reaction (ORR) in the polymer electrolyte membrane fuel cell ( PEMFC) is the main limitation for automotive applications. We demonstrated that the Pt3Ni( 111) surface is 10-fold more active for the ORR than the corresponding Pt(111) surface and 90-fold more active than the current state-of-the- art Pt/C catalysts for PEMFC. The Pt3Ni( 111) surface has an unusual electronic structure (d-band center position) and arrangement of surface atoms in the near-surface region. Under operating conditions relevant to fuel cells, its near-surface layer exhibits a highly structured compositional oscillation in the outermost and third layers, which are Pt-rich, and in the second atomic layer, which is Ni-rich. The weak interaction between the Pt surface atoms and nonreactive oxygenated species increases the number of active sites for O-2 adsorption.
Document Type: Article
Addresses: Argonne Natl Lab, Div Mat Sci, Argonne, IL 60439 USA.
Univ Calif Berkeley, Lawrence Berkeley Lab, Div Sci Mat, Berkeley, CA 94720 USA.
Univ Liverpool, Dept Phys, Oliver Lodge Lab, Liverpool L69 7ZE, Merseyside, England.
Univ S Carolina, Dept Chem & Phys, Aiken, SC 29801 USA.
Reprint Address: Stamenkovic, VR (reprint author), Argonne Natl Lab, Div Mat Sci, 9700 S Cass Ave, Argonne, IL 60439 USA.
E-mail Addresses: vrstamenkovic@anl.gov; nmmarkovic@anl.gov
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations
Author(s): Kovtyukhova, NI (Kovtyukhova, NI); Ollivier, PJ (Ollivier, PJ); Martin, BR (Martin, BR); Mallouk, TE (Mallouk, TE); Chizhik, SA (Chizhik, SA); Buzaneva, EV (Buzaneva, EV); Gorchinskiy, AD (Gorchinskiy, AD)
Source: CHEMISTRY OF MATERIALS Volume: 11 Issue: 3 Pages: 771-778 DOI: 10.1021/cm981085u Published: MAR 1999
Times Cited in Web of Science Core Collection: 2466
Total Times Cited: 2546
Abstract: Unilamellar colloids of graphite oxide (GO) were prepared from natural graphite and were grown as monolayer and multilayer thin films on cationic surfaces by electrostatic self-assembly. The multilayer films were grown by alternate adsorption of anionic GO sheets and cationic poly(allylamine hydrochloride) (PAH). The monolayer films consisted of 11-14 Angstrom thick GO sheets, with lateral dimensions between 150 nm and 9 mu m. Silicon substrates primed with amine monolayers gave partial GO monolayers, but surfaces primed with Al13O4-(OH)(24)(H2O)(12)(7+) ions gave densely tiled films that covered approximately 90% of the surface. When alkaline GO colloids were used, the monolayer assembly process selected the largest sheets (from 900 nm to 9 mu m) from the suspension. In this case, many of the flexible sheets appeared folded in AFM images. Multilayer (GO/PAH)(n) films were invariably thicker than expected from the individual thicknesses of the sheets and the polymer monolayers, and this behavior is also attributed to folding of the sheets. Multilayer (GO/PAH), and (GO/polyaniline)(n) films grown between indium-tin oxide and Pt electrodes show diodelike behavior, and higher currents are observed with the conductive polyaniline-containing films. The resisitivity of these films is decreased, as expected, by partial reduction of GO to carbon.
Document Type: Article
Addresses: Natl Acad Sci Ukraine, Inst Surface Chem, UA-252022 Kiev, Ukraine.
Penn State Univ, Dept Chem, University Pk, PA 16802 USA.
Met Polymer Res Inst, Gomel 246652, BELARUS.
Natl T Shevchenko Univ, UA-252033 Kiev, Ukraine.
Reprint Address: Kovtyukhova, NI (reprint author), Natl Acad Sci Ukraine, Inst Surface Chem, 31 Pr Nauky, UA-252022 Kiev, Ukraine.
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0897-4756
eISSN: 1520-5002
Title: Sorption of dye from aqueous solution by peat
Author(s): Ho, YS (Ho, YS); McKay, G (McKay, G)
Source: CHEMICAL ENGINEERING JOURNAL Volume: 70 Issue: 2 Pages: 115-124 DOI: 10.1016/S1385-8947(98)00076-X Published: JUN 1998
Times Cited in Web of Science Core Collection: 2354
Total Times Cited: 2459
Abstract: The sorption of two dyes, namely, Basic Blue 69 and Acid Blue 25 onto peat has been studied in terms of pseudo-second order and first order mechanisms for chemical sorption as well as an intraparticle diffusion mechanism process. The batch sorption process, based on the assumption of a pseudo-second order mechanism, has been developed to predict the rate constant of sorption, the equilibrium capacity and initial sorption rate with the effect of agitation, initial dye concentration and temperature. An activation energy of sorption has also been evaluated with the pseudo-second order rate constants. A comparison of the equilibrium sorption capacity evaluated has been made from pseudo-second order model and Langmuir isotherm. (C) 1998 Elsevier Science S.A. All rights reserved.
Document Type: Article
Addresses: Hong Kong Univ Sci & Technol, Dept Chem Engn, Hong Kong, Peoples R China.
Reprint Address: McKay, G (reprint author), Hong Kong Univ Sci & Technol, Dept Chem Engn, Clear Water Bay, Hong Kong, Peoples R China.
Web of Science Categories: Engineering, Environmental; Engineering, Chemical
ISSN: 1385-8947
Title: Renal clearance of quantum dots
Author(s): Choi, HS (Choi, Hak Soo); Liu, W (Liu, Wenhao); Misra, P (Misra, Preeti); Tanaka, E (Tanaka, Eiichi); Zimmer, JP (Zimmer, John P.); Ipe, BI (Ipe, Binil Itty); Bawendi, MG (Bawendi, Moungi G.); Frangioni, JV (Frangioni, John V.)
Source: NATURE BIOTECHNOLOGY Volume: 25 Issue: 10 Pages: 1165-1170 DOI: 10.1038/nbt1340 Published: OCT 2007
Times Cited in Web of Science Core Collection: 2345
Total Times Cited: 2372
Abstract: The field of nanotechnology holds great promise for the diagnosis and treatment of human disease. However, the size and charge of most nanoparticles preclude their efficient clearance from the body as intact nanoparticles. Without such clearance or their biodegradation into biologically benign components, toxicity is potentially amplified and radiological imaging is hindered. Using intravenously administered quantum dots in rodents as a model system, we have precisely defined the requirements for renal filtration and urinary excretion of inorganic, metal-containing nanoparticles. Zwitterionic or neutral organic coatings prevented adsorption of serum proteins, which otherwise increased hydrodynamic diameter by > 15 nm and prevented renal excretion. A final hydrodynamic diameter <5.5 nm resulted in rapid and efficient urinary excretion and elimination of quantum dots from the body. This study provides a foundation for the design and development of biologically targeted nanoparticles for biomedical applications.
Document Type: Article
Addresses: Beth Israel Deaconess Med Ctr, Dept Med, Div Hematol Oncol, Boston, MA 02215 USA.
Beth Israel Deaconess Med Ctr, Dept Radiol, Boston, MA 02215 USA.
MIT, Dept Chem, Cambridge, MA 02139 USA.
Reprint Address: Frangioni, JV (reprint author), Beth Israel Deaconess Med Ctr, Dept Med, Div Hematol Oncol, 330 Brookline Ave,Room SL-BO5, Boston, MA 02215 USA.
E-mail Addresses: mgb@mit.edu; jfrangio@bidmc.harvard.edu
Web of Science Categories: Biotechnology & Applied Microbiology
ISSN: 1087-0156
eISSN: 1546-1696
Title: Battery materials for ultrafast charging and discharging
Author(s): Kang, B (Kang, Byoungwoo); Ceder, G (Ceder, Gerbrand)
Source: NATURE Volume: 458 Issue: 7235 Pages: 190-193 DOI: 10.1038/nature07853 Published: MAR 12 2009
Times Cited in Web of Science Core Collection: 2327
Total Times Cited: 2395
Abstract: The storage of electrical energy at high charge and discharge rate is an important technology in today's society, and can enable hybrid and plug-in hybrid electric vehicles and provide back-up for wind and solar energy. It is typically believed that in electrochemical systems very high power rates can only be achieved with supercapacitors, which trade high power for low energy density as they only store energy by surface adsorption reactions of charged species on an electrode material(1-3). Here we show that batteries(4,5) which obtain high energy density by storing charge in the bulk of a material can also achieve ultrahigh discharge rates, comparable to those of supercapacitors. We realize this in LiFePO4 (ref. 6), a material with high lithium bulk mobility(7,8), by creating a fast ion-conducting surface phase through controlled off-stoichiometry. A rate capability equivalent to full battery discharge in 10-20 s can be achieved.
Document Type: Article
Addresses: [Kang, Byoungwoo; Ceder, Gerbrand] MIT, Dept Mat Sci & Engn, Cambridge, MA 02139 USA.
Reprint Address: Ceder, G (reprint author), MIT, Dept Mat Sci & Engn, 77 Massachusetts Ave, Cambridge, MA 02139 USA.
E-mail Addresses: gceder@mit.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
eISSN: 1476-4687
Title: Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells
Author(s): Nazeeruddin, MK (Nazeeruddin, MK); Pechy, P (Pechy, P); Renouard, T (Renouard, T); Zakeeruddin, SM (Zakeeruddin, SM); Humphry-Baker, R (Humphry-Baker, R); Comte, P (Comte, P); Liska, P (Liska, P); Cevey, L (Cevey, L); Costa, E (Costa, E); Shklover, V (Shklover, V); Spiccia, L (Spiccia, L); Deacon, GB (Deacon, GB); Bignozzi, CA (Bignozzi, CA); Gratzel, M (Gratzel, M)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 123 Issue: 8 Pages: 1613-1624 DOI: 10.1021/ja003299u Published: FEB 28 2001
Times Cited in Web of Science Core Collection: 2184
Total Times Cited: 2237
Abstract: A new series of panchromatic ruthenium(II) sensitizers derived from carboxylated terpyridyl complexes of tris-thiocyanato Ru(II) have been developed. Black dye containing different degrees of protonation {(C2H5)(3)NH}[Ru(H(3)tcterpy)(NCS)(3)] 1, {(C4H9)(4)N}(2)[Ru(H(2)tcterpy)(NCS)(3)] 2, {(C4H9)(4)N}(3)[RU(Htcterpy)(NCS)(3)] 3, and {(C4H9)(4)N}(4)[Ru(tcterpy)(NCS)(3)] 4 (tcterpy = 4,4',4 " -tricarboxy-2,2':6',2 " -terpyridine) have been synthesized and fully characterized by UV-vis, emission, IR, Raman, NMR, cyclic voltammetry, and X-ray diffraction studies. The crystal structure of complex 2 confirms the presence of a Ru(II)N6 central core derived from the terpyridine ligand and three N-bonded thiocyanates. Intermolecular H-bonding between carboxylates on neighboring terpyridines gives rise to 2-D H-bonded arrays. The absorption and emission maxima of the black dye show a bathochromic shift with decreasing pH and exhibit pH-dependent excited-state lifetimes. The red-shift of the emission maxima is due to better pi -acceptor properties of the acid form that lowers the energy of the CT excited state. The low-energy metal-to-ligand charge-transfer absorption band showed marked solvatochromism due to the presence of thiocyanate ligands. The Ru(II)/(III) oxidation potential of the black dye and the ligand-based reduction potential shifted cathodically with decreasing number of protons and showed more reversible character. The adsorption of complex 3 from methoxyacetonitrile solution onto transparent TiO2 films was interpreted by a Langmuir isotherm yielding an adsorption equilibrium constant, K-ads, of (1.0 +/- 0.3) x 10(5) M-1. The amount of dye adsorbed at monolayer saturation was (n(alpha) = 6.9 +/- 0.3) x 10(-8) mol/mg of TiO2, which is around 30% less than that of the cis-di(thiocyanato)bis(2,2'-bipyridyl-4,4'-dicarboxylate)-ruthenium(II) complex. The black dye, when anchored to nanocrystalline;TiO2. films achieves very efficient sensitization over the whole visible range extending into the near-IR region up to 920 nm, yielding over 80% incident photon to-current efficiencies (IPCE). solar cells containing the black dye were subjected to analysis by a photovoltaic calibration laboratory (NREL, U.S.A.) to determine their solar-to-electric conversion efficiency under standard AM 1.5 sunlight. A short circuit photocurrent density obtained was 20.5 mA/cm(2), and the open circuit voltage was 0.72 V corresponding to an overall conversion efficiency of 10.4%.
Document Type: Article
Addresses: Swiss Fed Inst Technol, Lab Photon & Interfaces, Inst Phys Chem, CH-1015 Lausanne, Switzerland.
Reprint Address: Nazeeruddin, MK (reprint author), Swiss Fed Inst Technol, Lab Photon & Interfaces, Inst Phys Chem, CH-1015 Lausanne, Switzerland.
E-mail Addresses: Mdkhaja.Nazeeruddin@epfl.ch
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: BUILDUP OF ULTRATHIN MULTILAYER FILMS BY A SELF-ASSEMBLY PROCESS .3. CONSECUTIVELY ALTERNATING ADSORPTION OF ANIONIC AND CATIONIC POLYELECTROLYTES ON CHARGED SURFACES
Author(s): DECHER, G (DECHER, G); HONG, JD (HONG, JD); SCHMITT, J (SCHMITT, J)
Source: THIN SOLID FILMS Volume: 210 Issue: 1-2 Pages: 831-835 DOI: 10.1016/0040-6090(92)90417-A Published: APR 30 1992
Times Cited in Web of Science Core Collection: 2325
Total Times Cited: 2396
Abstract: A solid substrate with a positively charged planar surface is immersed in a solution containing an anionic polyelectrolyte and a monolayer of the polyanion is adsorbed. Since the adsorption is carried out at relatively high concentrations of polyelectrolyte, a large number of ionic residues remain exposed to the interface with the solution and thus the surface charge is effectively reversed. After rinsing in pure water the substrate is immersed in the solution containing a cationic polyelectrolyte. Again a monolayer is adsorbed but now the original surface charge is restored. By repeating both steps in a cyclic fashion, alternating multilayer assemblies of both polymers are obtained. The buildup of the multilayer films was followed by UV/vis spectroscopy and small angle X-ray scattering (SAXS). It is demonstrated that multilayer films composed of at least 100 consecutively alternating layers can be assembled.
Document Type: Article; Proceedings Paper
Conference Title: 5TH INTERNATIONAL CONF ON LANGMUIR-BLODGETT FILMS
Conference Date: AUG 26-30, 1991
Conference Location: PARIS, FRANCE
Reprint Address: DECHER, G (reprint author), UNIV MAINZ,INST PHYS CHEM,W-6500 MAINZ,GERMANY.
Web of Science Categories: Materials Science, Multidisciplinary; Materials Science, Coatings & Films; Physics, Applied; Physics, Condensed Matter
ISSN: 0040-6090
Title: STUDIES IN ADSORPTION .11. A SYSTEM OF CLASSIFICATION OF SOLUTION ADSORPTION ISOTHERMS, AND ITS USE IN DIAGNOSIS OF ADSORPTION MECHANISMS AND IN MEASUREMENT OF SPECIFIC SURFACE AREAS OF SOLIDS
Author(s): GILES, CH (GILES, CH); MACEWAN, TH (MACEWAN, TH); NAKHWA, SN (NAKHWA, SN); SMITH, D (SMITH, D)
Source: JOURNAL OF THE CHEMICAL SOCIETY Issue: OCT Pages: 3973-3993 DOI: 10.1039/jr9600003973 Published: 1960
Times Cited in Web of Science Core Collection: 2292
Total Times Cited: 2338
Document Type: Article
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0368-1769
Title: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)
Author(s): Thommes, M (Thommes, Matthias); Kaneko, K (Kaneko, Katsumi); Neimark, AV (Neimark, Alexander V.); Olivier, JP (Olivier, James P.); Rodriguez-Reinoso, F (Rodriguez-Reinoso, Francisco); Rouquerol, J (Rouquerol, Jean); Sing, KSW (Sing, Kenneth S. W.)
Source: PURE AND APPLIED CHEMISTRY Volume: 87 Issue: 9-10 Pages: 1051-1069 DOI: 10.1515/pac-2014-1117 Published: OCT 2015
Times Cited in Web of Science Core Collection: 2272
Total Times Cited: 2298
Abstract: Gas adsorption is an important tool for the characterisation of porous solids and fine powders. Major advances in recent years have made it necessary to update the 1985 IUPAC manual on Reporting Physisorption Data for Gas/Solid Systems. The aims of the present document are to clarify and standardise the presentation, nomenclature and methodology associated with the application of physisorption for surface area assessment and pore size analysis and to draw attention to remaining problems in the interpretation of physisorption data.
Document Type: Article
Addresses: [Thommes, Matthias] Quantachrome Instruments, Dept Appl Sci, Boynton Beach, FL 33426 USA.
[Kaneko, Katsumi] Shinshu Univ, Ctr Energy & Environm Sci, Nagano, Japan.
[Neimark, Alexander V.] Rutgers State Univ, Dept Chem & Biochem Engn, Piscataway, NJ USA.
[Olivier, James P.] Micromerit Instrument Corp, Norcross, NJ USA.
[Rodriguez-Reinoso, Francisco] Univ Alicante, Dept Quim Inorgan, Lab Mat Avanzados, E-03080 Alicante, Spain.
[Rouquerol, Jean] Aix Marseille Univ, Lab MADIREL, Ctr St Jerome, Marseilles, France.
[Sing, Kenneth S. W.] Brunel Univ, London, England.
Reprint Address: Thommes, M (reprint author), Quantachrome Instruments, Dept Appl Sci, 1900 Corp Dr, Boynton Beach, FL 33426 USA.
E-mail Addresses: matthias.thommes@quantachrome.com
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0033-4545
eISSN: 1365-3075
Title: POLYMER ELECTROLYTE FUEL-CELL MODEL
Author(s): SPRINGER, TE (SPRINGER, TE); ZAWODZINSKI, TA (ZAWODZINSKI, TA); GOTTESFELD, S (GOTTESFELD, S)
Source: JOURNAL OF THE ELECTROCHEMICAL SOCIETY Volume: 138 Issue: 8 Pages: 2334-2342 DOI: 10.1149/1.2085971 Published: AUG 1991
Times Cited in Web of Science Core Collection: 2171
Total Times Cited: 2239
Abstract: We present here an isothermal, one-dimensional, steady-state model for a complete polymer electrolyte fuel cell (PEFC) with a 117 Nafion(R) membrane. In this model we employ water diffusion coefficients electro-osmotic drag coefficients, water sorption isotherms, and membrane conductivities, all measured in our laboratory as functions of membrane water content. The model predicts a net-water-per-proton flux ratio of 0.2 H2O/H+ under typical operating conditions, which is much less than the measured electro-osmotic drag coefficient for a fully hydrated membrane. It also predicts an increase in membrane resistance with increased current density and demonstrates the great advantage of a thinner membrane in alleviating this resistance problem. Both of these predictions were verified experimentally under certain conditions.
Document Type: Article
Reprint Address: SPRINGER, TE (reprint author), UNIV CALIF LOS ALAMOS SCI LAB,LOS ALAMOS,NM 87545, USA.
Web of Science Categories: Electrochemistry; Materials Science, Coatings & Films
ISSN: 0013-4651
Title: Tin-based amorphous oxide: A high-capacity lithium-ion-storage material
Author(s): Idota, Y (Idota, Y); Kubota, T (Kubota, T); Matsufuji, A (Matsufuji, A); Maekawa, Y (Maekawa, Y); Miyasaka, T (Miyasaka, T)
Source: SCIENCE Volume: 276 Issue: 5317 Pages: 1395-1397 DOI: 10.1126/science.276.5317.1395 Published: MAY 30 1997
Times Cited in Web of Science Core Collection: 2149
Total Times Cited: 2222
Abstract: A high-capacity lithium-storage material in metal-oxide form has been synthesized that can replace the carbon-based lithium intercalation materials currently in extensive use as the negative electrode (anode) of lithium-ion rechargeable batteries. This tin-based amorphous composite oxide (TCO) contains Sn(II)-O as the active center for lithium insertion and other glass-forming elements, which make up an oxide network. The TCO anode yields a specific capacity for reversible lithium adsorption more than 50 percent higher than those of the carbon families that persists after charge-discharge cycling when coupled with a lithium cobalt oxide cathode. Lithium-7 nuclear magnetic resonance measurements evidenced the high ionic state of lithium retained in the charged state, in which TCO accepted 8 moles of lithium ions per unit mole.
Document Type: Article
Addresses: FUJI PHOTO FILM CO LTD,ASHIGARA RES LABS,KANAGAWA 25001,JAPAN.
FUJIFILM CELLTEC,TAIWA,MIYAGI 98134,JAPAN.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points
Author(s): Henkelman, G (Henkelman, G); Jonsson, H (Jonsson, H)
Source: JOURNAL OF CHEMICAL PHYSICS Volume: 113 Issue: 22 Pages: 9978-9985 Article Number: PII [S0021-9606(00)70546-0] DOI: 10.1063/1.1323224 Published: DEC 8 2000
Times Cited in Web of Science Core Collection: 2136
Total Times Cited: 2152
Abstract: An improved way of estimating the local tangent in the nudged elastic band method for finding minimum energy paths is presented. In systems where the force along the minimum energy path is large compared to the restoring force perpendicular to the path and when many images of the system are included in the elastic band, kinks can develop and prevent the band from converging to the minimum energy path. We show how the kinks arise and present an improved way of estimating the local tangent which solves the problem. The task of finding an accurate energy and configuration for the saddle point is also discussed and examples given where a complementary method, the dimer method, is used to efficiently converge to the saddle point. Both methods only require the first derivative of the energy and can, therefore, easily be applied in plane wave based density-functional theory calculations. Examples are given from studies of the exchange diffusion mechanism in a Si crystal, Al addimer formation on the Al(100) surface, and dissociative adsorption of CH4 on an Ir(111) surface. (C) 2000 American Institute of Physics. [S0021-9606(00)70546-0].
Document Type: Article
Addresses: Univ Washington, Dept Chem, Seattle, WA 98195 USA.
Reprint Address: Henkelman, G (reprint author), Univ Washington, Dept Chem, Box 351700, Seattle, WA 98195 USA.
E-mail Addresses: graeme@u.washington.edu; hannes@u.washington.edu
Web of Science Categories: Chemistry, Physical; Physics, Atomic, Molecular & Chemical
ISSN: 0021-9606
eISSN: 1089-7690
Title: Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer
Author(s): Chmiola, J (Chmiola, J.); Yushin, G (Yushin, G.); Gogotsi, Y (Gogotsi, Y.); Portet, C (Portet, C.); Simon, P (Simon, P.); Taberna, PL (Taberna, P. L.)
Source: SCIENCE Volume: 313 Issue: 5794 Pages: 1760-1763 DOI: 10.1126/science.1132195 Published: SEP 22 2006
Times Cited in Web of Science Core Collection: 2124
Total Times Cited: 2165
Abstract: Carbon supercapacitors, which are energy storage devices that use ion adsorption on the surface of highly porous materials to store charge, have numerous advantages over other power-source technologies, but could realize further gains if their electrodes were properly optimized. Studying the effect of the pore size on capacitance could potentially improve performance by maximizing the electrode surface area accessible to electrolyte ions, but until recently, no studies had addressed the lower size limit of accessible pores. Using carbide-derived carbon, we generated pores with average sizes from 0.6 to 2.25 nanometer and studied double-layer capacitance in an organic electrolyte. The results challenge the long-held axiom that pores smaller than the size of solvated electrolyte ions are incapable of contributing to charge storage.
Document Type: Article
Addresses: Drexel Univ, Dept Mat Sci & Engn, Philadelphia, PA 19104 USA.
Drexel Univ, AJ Drexel Nanotechnol Inst, Philadelphia, PA 19104 USA.
Univ Toulouse 3, CIRIMAT, CNRS, UMR 5085, F-31062 Toulouse 4, France.
Reprint Address: Gogotsi, Y (reprint author), Drexel Univ, Dept Mat Sci & Engn, Philadelphia, PA 19104 USA.
E-mail Addresses: gogotsi@drexel.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
eISSN: 1095-9203
Title: THERMODYNAMICS OF MIXED-GAS ADSORPTION
Author(s): MYERS, AL (MYERS, AL); PRAUSNITZ, JM (PRAUSNITZ, JM)
Source: AICHE JOURNAL Volume: 11 Issue: 1 Pages: 121-+ DOI: 10.1002/aic.690110125 Published: 1965
Times Cited in Web of Science Core Collection: 2119
Total Times Cited: 2152
Document Type: Article
Web of Science Categories: Engineering, Chemical
ISSN: 0001-1541
Title: THE ADSORPTION OF PROTEINS ON ERYTHROCYTES TREATED WITH TANNIC ACID AND SUBSEQUENT HEMAGGLUTINATION BY ANTIPROTEIN SERA
Author(s): BOYDEN, SV (BOYDEN, SV)
Source: JOURNAL OF EXPERIMENTAL MEDICINE Volume: 93 Issue: 2 Pages: 107-120 DOI: 10.1084/jem.93.2.107 Published: 1951
Times Cited in Web of Science Core Collection: 2087
Total Times Cited: 2093
Document Type: Article
Web of Science Categories: Immunology; Medicine, Research & Experimental
ISSN: 0022-1007
Title: Ultrahigh Porosity in Metal-Organic Frameworks
Author(s): Furukawa, H (Furukawa, Hiroyasu); Ko, N (Ko, Nakeun); Go, YB (Go, Yong Bok); Aratani, N (Aratani, Naoki); Choi, SB (Choi, Sang Beom); Choi, E (Choi, Eunwoo); Yazaydin, AO (Yazaydin, A. Oezguer); Snurr, RQ (Snurr, Randall Q.); O'Keeffe, M (O'Keeffe, Michael); Kim, J (Kim, Jaheon); Yaghi, OM (Yaghi, Omar M.)
Source: SCIENCE Volume: 329 Issue: 5990 Pages: 424-428 DOI: 10.1126/science.1192160 Published: JUL 23 2010
Times Cited in Web of Science Core Collection: 2041
Total Times Cited: 2083
Abstract: Crystalline solids with extended non-interpenetrating three-dimensional crystal structures were synthesized that support well-defined pores with internal diameters of up to 48 angstroms. The Zn(4)O(CO(2))(6) unit was joined with either one or two kinds of organic link, 4,4',4 ''-[benzene-1,3,5-triyl-tris (ethyne-2,1-diyl)]tribenzoate (BTE), 4,4',44 ''-[benzene-1,3,5-triyl-tris(benzene-4,1-diyl)]tribenzoate (BBC), 4,4',44 ''-benzene-1,3,5-triyl-tribenzoate (BTB)/2,6-naphthalenedicarboxylate (NDC), and BTE/biphenyl-4,4'-dicarboxylate (BPDC), to give four metal-organic frameworks (MOFs), MOF-180, -200, -205, and -210, respectively. Members of this series of MOFs show exceptional porosities and gas (hydrogen, methane, and carbon dioxide) uptake capacities. For example, MOF-210 has Brunauer-Emmett-Teller and Langmuir surface areas of 6240 and 10,400 square meters per gram, respectively, and a total carbon dioxide storage capacity of 2870 milligrams per gram. The volume-specific internal surface area of MOF-210 (2060 square meters per cubic centimeter) is equivalent to the outer surface of nanoparticles (3-nanometer cubes) and near the ultimate adsorption limit for solid materials.
Document Type: Article
Addresses: [Ko, Nakeun; Choi, Sang Beom; Kim, Jaheon] Soongsil Univ, Dept Chem, Seoul 156743, South Korea.
[Furukawa, Hiroyasu; Go, Yong Bok; Aratani, Naoki; Choi, Eunwoo; O'Keeffe, Michael; Yaghi, Omar M.] Univ Calif Los Angeles, Calif NanoSyst Inst, Ctr Reticular Chem, Los Angeles, CA 90095 USA.
[Furukawa, Hiroyasu; Go, Yong Bok; Aratani, Naoki; Choi, Eunwoo; O'Keeffe, Michael; Yaghi, Omar M.] Univ Calif Los Angeles, Dept Chem & Biochem, Los Angeles, CA 90095 USA.
[Yazaydin, A. Oezguer; Snurr, Randall Q.] Northwestern Univ, Dept Chem & Biol Engn, Evanston, IL 60208 USA.
[Yaghi, Omar M.] Univ Calif Los Angeles, Inst Genom & Prote, Dept Energy, Los Angeles, CA 90095 USA.
Reprint Address: Kim, J (reprint author), Soongsil Univ, Dept Chem, Seoul 156743, South Korea.
E-mail Addresses: jaheon@ssu.ac.kr; yaghi@chem.ucla.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: SORPTION OF HYDROPHOBIC POLLUTANTS ON NATURAL SEDIMENTS
Author(s): KARICKHOFF, SW (KARICKHOFF, SW); BROWN, DS (BROWN, DS); SCOTT, TA (SCOTT, TA)
Source: WATER RESEARCH Volume: 13 Issue: 3 Pages: 241-248 DOI: 10.1016/0043-1354(79)90201-X Published: 1979
Times Cited in Web of Science Core Collection: 2004
Total Times Cited: 2077
Document Type: Article
Reprint Address: KARICKHOFF, SW (reprint author), US EPA,ENVIRONM RES LAB,COLL STN RD,ATHENS,GA 30605, USA.
Web of Science Categories: Engineering, Environmental; Environmental Sciences; Water Resources
ISSN: 0043-1354
Title: The kinetics of sorption of divalent metal ions onto sphagnum moss peat
Author(s): Ho, YS (Ho, YS); McKay, G (McKay, G)
Source: WATER RESEARCH Volume: 34 Issue: 3 Pages: 735-742 DOI: 10.1016/S0043-1354(99)00232-8 Published: FEB 2000
Times Cited in Web of Science Core Collection: 1990
Total Times Cited: 2107
Abstract: A pseudo-second order rate equation describing the kinetics of sorption of divalent metal ions onto sphagnum moss peat at different initial metal ion concentrations and pear doses has been developed. The kinetics of sorption were followed based on the amounts of metal sorbed at various time intervals. Results show that sorption (chemical bonding) might be rate-limiting in the sorption of divalent metal ions onto peat during agitated batch contact time experiments. The rate constant, the equilibrium sorption capacity and the initial sorption rate were calculated. From these parameters, an empirical model for predicting the sorption capacity of metal ions sorbed was derived. (C) 2000 Elsevier Science Ltd. All rights reserved.
Document Type: Article
Addresses: Hong Kong Univ Sci & Technol, Dept Chem Engn, Kowloon, Peoples R China.
Reprint Address: McKay, G (reprint author), Hong Kong Univ Sci & Technol, Dept Chem Engn, Clear Water Bay, Kowloon, Peoples R China.
Web of Science Categories: Engineering, Environmental; Environmental Sciences; Water Resources
ISSN: 0043-1354
Title: PORE- AND SOLID-DIFFUSION KINETICS IN FIXED-BED ADSORPTION UNDER CONSTANT-PATTERN CONDITIONS
Author(s): HALL, KR (HALL, KR); EAGLETON, LC (EAGLETON, LC); ACRIVOS, A (ACRIVOS, A); VERMEULEN, T (VERMEULEN, T)
Source: INDUSTRIAL & ENGINEERING CHEMISTRY FUNDAMENTALS Volume: 5 Issue: 2 Pages: 212-+ DOI: 10.1021/i160018a011 Published: 1966
Times Cited in Web of Science Core Collection: 1969
Total Times Cited: 2022
Document Type: Article
Web of Science Categories: Engineering, Chemical; Engineering, Industrial
ISSN: 0196-4313
Title: THE EXCHANGE ADSORPTION OF IONS FROM AQUEOUS SOLUTIONS BY ORGANIC ZEOLITES .2.
Author(s): BOYD, GE (BOYD, GE); ADAMSON, AW (ADAMSON, AW); MYERS, LS (MYERS, LS)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 69 Issue: 11 Pages: 2836-2848 DOI: 10.1021/ja01203a066 Published: 1947
Times Cited in Web of Science Core Collection: 1949
Total Times Cited: 2029
Document Type: Article
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Van der Waals density functionals applied to solids
Author(s): Klimes, J (Klimes, Jiri); Bowler, DR (Bowler, David R.); Michaelides, A (Michaelides, Angelos)
Source: PHYSICAL REVIEW B Volume: 83 Issue: 19 Article Number: 195131 DOI: 10.1103/PhysRevB.83.195131 Published: MAY 25 2011
Times Cited in Web of Science Core Collection: 1947
Total Times Cited: 1954
Abstract: The van der Waals density functional (vdW-DF) of M. Dion et al. [Phys. Rev. Lett. 92, 246401 (2004)] is a promising approach for including dispersion in approximate density functional theory exchange-correlation functionals. Indeed, an improved description of systems held by dispersion forces has been demonstrated in the literature. However, despite many applications, standard general tests on a broad range of materials including traditional "hard" matter such asmetals, ionic compounds, and insulators are lacking. Such tests are important not least because many of the applications of the vdW-DF method focus on the adsorption of atoms and molecules on the surfaces of solids. Here we calculate the lattice constants, bulk moduli, and atomization energies for a range of solids using the original vdW-DF and several of its offspring. We find that the original vdW-DF overestimates lattice constants in a similar manner to how it overestimates binding distances for gas-phase dimers. However, some of the modified vdW functionals lead to average errors which are similar to those of PBE or better. Likewise, atomization energies that are slightly better than from PBE are obtained from the modified vdW-DFs. Although the tests reported here are for hard solids, not normally materials for which dispersion forces are thought to be important, we find a systematic improvement in cohesive properties for the alkali metals and alkali halides when nonlocal correlations are accounted for.
Document Type: Article
Addresses: [Klimes, Jiri; Bowler, David R.; Michaelides, Angelos] UCL, London Ctr Nanotechnol, London WC1E 6BT, England.
[Klimes, Jiri; Michaelides, Angelos] UCL, Dept Chem, London WC1E 6BT, England.
[Bowler, David R.] UCL, Dept Phys & Astron, London WC1E 6BT, England.
Reprint Address: Klimes, J (reprint author), UCL, London Ctr Nanotechnol, London WC1E 6BT, England.
E-mail Addresses: angelos.michaelides@ucl.ac.uk
Web of Science Categories: Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1098-0121
Title: Modification of the surface chemistry of activated carbons
Author(s): Figueiredo, JL (Figueiredo, JL); Pereira, MFR (Pereira, MFR); Freitas, MMA (Freitas, MMA); Orfao, JJM (Orfao, JJM)
Source: CARBON Volume: 37 Issue: 9 Pages: 1379-1389 DOI: 10.1016/S0008-6223(98)00333-9 Published: 1999
Times Cited in Web of Science Core Collection: 1900
Total Times Cited: 1979
Abstract: A NORIT activated carbon was modified by different chemical and thermal treatments (including oxidation in the gas and liquid phases) in order to obtain materials with different surface properties. Several techniques were used to characterize these materials including nitrogen adsorption, chemical and thermal analyses, XPS, TPD and DRIFTS. The results obtained by TPD agree quantitatively with the elemental and proximate analyses of the oxidized materials, and qualitatively with the observations by DRIFTS. A simple deconvolution method is proposed to analyse the TPD spectra, allowing for the quantitative determination of the amount of each functional group on the surface. A multiple gaussian function has been shown to fit the data adequately, the parameters obtained for each fit matching very well the features observed in the experimentally determined TPD spectra.
It is shown that gas phase oxidation of the carbon increases mainly the concentration of hydroxyl and carbonyl surface groups, while oxidations in the liquid phase increase especially the concentration of carboxylic acids. (C) 1999 Elsevier Science Ltd. All rights reserved.
Document Type: Article
Addresses: Univ Porto, Fac Engn, Dept Engn Quim, Lab Catalise & Mat, P-4099 Oporto, Portugal.
Reprint Address: Figueiredo, JL (reprint author), Univ Porto, Fac Engn, Dept Engn Quim, Lab Catalise & Mat, P-4099 Oporto, Portugal.
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0008-6223
Title: Polymer-layered silicate nanocomposites: an overview
Author(s): LeBaron, PC (LeBaron, PC); Wang, Z (Wang, Z); Pinnavaia, TJ (Pinnavaia, TJ)
Source: APPLIED CLAY SCIENCE Volume: 15 Issue: 1-2 Pages: 11-29 DOI: 10.1016/S0169-1317(99)00017-4 Published: SEP 1999
Times Cited in Web of Science Core Collection: 1897
Total Times Cited: 1953
Abstract: An overview of polymer-clay hybrid nanocomposites is provided with emphasis placed on the use of alkylammonium exchanged smectite clays as the reinforcement phase in selected polymer matrices. A few weight percent loading of organoclay in nylon 6 boosts the heat distortion temperature by 80 degrees C, making possible structural applications under conditions where the pristine polymer would normally fail. A similar loading of clay nanolayers in elastomeric epoxy and polyurethane matrices dramatically improves bath the toughness and the tensile properties of these thermoset systems. Glassy epoxy nanocomposites exhibit substantial improvement in yield strength and modulus under compressive stress-strain conditions. The latest development in polypropylene hybrids have yielded nanocomposites with improved storage moduli. Polyimide hybrids in thin-film form display a 10-fold decrease in permeability toward water vapor at 2 wt.% clay loading. In situ and melt intercalation processing methods are effective in producing reinforced polystyrene hybrids. Nitrile rubber hybrids show improved storage moduli and reduced permeabilities even toward gases as small as hydrogen. Poly(epsilon-caprolactone)-clay nanocomposites prepared by in situ polymerization of epsilon-caprolactone in organoclay galleries show a substantial reduction in water adsorption. Polysiloxane nanocomposites produced from poly(dimethylsiloxane) and organoclay mixtures have improved in tensile properties, thermal stability and resistance to swelling solvents. Organoclay-poly(l-lactide) composite film was obtained by solvent casting technique. Clay nanolayers dispersed in liquid crystals act as structure directors and form hybrids composites that can be switched from being highly opaque to highly transparent by applying an electric field of short duration. (C) 1999 Published by Elsevier Science B.V.
Document Type: Article
Addresses: Michigan State Univ, Dept Chem, Funct Mat Res Ctr, E Lansing, MI 48824 USA.
Michigan State Univ, Ctr Composite Mat & Struct, E Lansing, MI 48824 USA.
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary; Mineralogy
ISSN: 0169-1317
Title: Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces
Author(s): Stamenkovic, VR (Stamenkovic, Vojislav R.); Mun, BS (Mun, Bongjin Simon); Arenz, M (Arenz, Matthias); Mayrhofer, KJJ (Mayrhofer, Karl J. J.); Lucas, CA (Lucas, Christopher A.); Wang, GF (Wang, Guofeng); Ross, PN (Ross, Philip N.); Markovic, NM (Markovic, Nenad M.)
Source: NATURE MATERIALS Volume: 6 Issue: 3 Pages: 241-247 DOI: 10.1038/nmat1840 Published: MAR 2007
Times Cited in Web of Science Core Collection: 1863
Total Times Cited: 1880
Abstract: One of the key objectives in fuel-cell technology is to improve and reduce Pt loading as the oxygen-reduction catalyst. Here, we show a fundamental relationship in electrocatalytic trends on Pt3M (M = Ni, Co, Fe, Ti, V) surfaces between the experimentally determined surface electronic structure (the d-band centre) and activity for the oxygen-reduction reaction. This relationship exhibits 'volcano-type' behaviour, where the maximum catalytic activity is governed by a balance between adsorption energies of reactive intermediates and surface coverage by spectator (blocking) species. The electrocatalytic trends established for extended surfaces are used to explain the activity pattern of Pt3M nanocatalysts as well as to provide a fundamental basis for the catalytic enhancement of cathode catalysts. By combining simulations with experiments in the quest for surfaces with desired activity, an advanced concept in nanoscale catalyst engineering has been developed.
Document Type: Article
Addresses: Argonne Natl Lab, Div Mat Sci, Argonne, IL 60439 USA.
Univ Calif Berkeley, Lawrence Berkeley Lab, Div Mat Sci, Berkeley, CA 94720 USA.
Hanyang Univ, Dept Appl Phys, Ansan 426791, Kyunggi Do, South Korea.
Tech Univ Munich, D-80333 Munich, Germany.
Univ Liverpool, Dept Phys, Oliver Lodge Lab, Liverpool L69 7ZE, Merseyside, England.
Univ S Carolina, Dept Chem & Phys, Aiken, SC 29801 USA.
Reprint Address: Stamenkovic, VR (reprint author), Argonne Natl Lab, Div Mat Sci, 9700 S Cass Ave, Argonne, IL 60439 USA.
E-mail Addresses: vrstamenkovic@anl.gov; nmmarkovic@anl.gov
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1476-1122
eISSN: 1476-4660
Title: COMPARISON OF THE STRUCTURES AND WETTING PROPERTIES OF SELF-ASSEMBLED MONOLAYERS OF NORMAL-ALKANETHIOLS ON THE COINAGE METAL-SURFACES, CU, AG, AU
Author(s): LAIBINIS, PE (LAIBINIS, PE); WHITESIDES, GM (WHITESIDES, GM); ALLARA, DL (ALLARA, DL); TAO, YT (TAO, YT); PARIKH, AN (PARIKH, AN); NUZZO, RG (NUZZO, RG)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 113 Issue: 19 Pages: 7152-7167 DOI: 10.1021/ja00019a011 Published: SEP 11 1991
Times Cited in Web of Science Core Collection: 1790
Total Times Cited: 1800
Abstract: Long-chain alkanethiols, HS(CH2)nCH3, adsorb from solution onto the surfaces of gold, silver, and copper and form monolayers. Reflection infrared spectroscopy indicates that monolayers on silver and on copper (when carefully prepared) have the chains in well-defined molecular orientations and in crystalline-like phase states, as has been observed on gold. Monolayers on silver are structurally related to those formed by adsorption on gold, but different in details of orientation. The monolayers formed on copper are structurally more complex and show a pronounced sensitivity to the details of the sample preparation. Quantitative analysis of the IR data using numerical simulations based on an average single chain model suggests that the alkyl chains in monolayers on silver are all-trans zig-zag and canted by approximately 12-degrees from the normal to the surface. The analysis also suggests a twist of the plane containing the carbon backbone of approximately 45-degrees from the plane defined by the tilt and surface normal vectors. For comparison, the monolayers that form on adsorption of alkanethiols on gold surfaces, as judged by their vibrational spectra, are also trans zig-zag extended but, when interpreted in the context of the same single chain model, have a cant angle of approximately 27-degrees and a twist of the plane of the carbon backbone of approximately 53-degrees. The monolayers formed on copper (when they are obtained in high quality) exhibit infrared spectra effectively indistinguishable from those on silver and thus appear to have the same structure. Films on copper are also commonly obtained that are structurally ill-defined and appear to contain significant densities of gauche conformations. These spectroscopically based interpretations are compatible with inferences from wetting and XPS measurements. The structure of the substrate-sulfur interface appears to control molecular orientations of the alkyl groups in these films. An improved structural model, incorporating a two-chain unit cell and allowing for the temperature-dependent population of gauche conformations, is presented and applied to the specific case of the structures formed on gold.
Document Type: Article
Addresses: ACAD SINICA,INST CHEM,TAIPEI 115,TAIWAN.
UNIV ILLINOIS,DEPT CHEM,URBANA,IL 61801.
UNIV ILLINOIS,DEPT MAT SCI,URBANA,IL 61801.
HARVARD UNIV,DEPT CHEM,CAMBRIDGE,MA 02138.
PENN STATE UNIV,DEPT MAT SCI,UNIVERSITY PK,PA 16802.
AT&T BELL LABS,MURRAY HILL,NJ 07974.
PENN STATE UNIV,DEPT CHEM,UNIVERSITY PK,PA 16802.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: SELF-ASSEMBLED ORGANIC MONOLAYERS - MODEL SYSTEMS FOR STUDYING ADSORPTION OF PROTEINS AT SURFACES
Author(s): PRIME, KL (PRIME, KL); WHITESIDES, GM (WHITESIDES, GM)
Source: SCIENCE Volume: 252 Issue: 5009 Pages: 1164-1167 DOI: 10.1126/science.252.5009.1164 Published: MAY 24 1991
Times Cited in Web of Science Core Collection: 1785
Total Times Cited: 1806
Abstract: Self-assembled monolayers (SAMs) of omega-functionalized long-chain alkanethiolates on gold films are excellent model systems with which to study the interactions of proteins with organic surfaces. Monolayers containing mixtures of hydrophobic (methyl-terminated) and hydrophilic [hydroxyl-, maltose-, and hexa(ethylene glycol)-terminated] alkanethiols can be tailored to select specific degrees of adsorption: the amount of protein adsorbed varies monotonically with the composition of the monolayer. The hexa(ethylene glycol)-terminated SAMs are the most effective in resisting protein adsorption. The ability to create interfaces with similar structures and well-defined compositions should make it possible to test hypotheses concerning protein adsorption.
Document Type: Article
Addresses: HARVARD UNIV,DEPT CHEM,CAMBRIDGE,MA 02138.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: Doping graphene with metal contacts
Author(s): Giovannetti, G (Giovannetti, G.); Khomyakov, PA (Khomyakov, P. A.); Brocks, G (Brocks, G.); Karpan, VM (Karpan, V. M.); van den Brink, J (van den Brink, J.); Kelly, PJ (Kelly, P. J.)
Source: PHYSICAL REVIEW LETTERS Volume: 101 Issue: 2 Article Number: 026803 DOI: 10.1103/PhysRevLett.101.026803 Published: JUL 11 2008
Times Cited in Web of Science Core Collection: 1727
Total Times Cited: 1740
Abstract: Making devices with graphene necessarily involves making contacts with metals. We use density functional theory to study how graphene is doped by adsorption on metal substrates and find that weak bonding on Al, Ag, Cu, Au, and Pt, while preserving its unique electronic structure, can still shift the Fermi level with respect to the conical point by similar to 0.5 eV. At equilibrium separations, the crossover from p-type to n-type doping occurs for a metal work function of similar to 5.4 eV, a value much larger than the graphene work function of 4.5 eV. The numerical results for the Fermi level shift in graphene are described very well by a simple analytical model which characterizes the metal solely in terms of its work function, greatly extending their applicability.
Document Type: Article
Addresses: [Giovannetti, G.; van den Brink, J.] Leiden Univ, Inst Lorentz Theoret Phys, NL-2300 RA Leiden, Netherlands.
[Giovannetti, G.; Khomyakov, P. A.; Brocks, G.; Karpan, V. M.; Kelly, P. J.] Univ Twente, Fac Sci & Technol, NL-7500 AE Enschede, Netherlands.
[Giovannetti, G.; Khomyakov, P. A.; Brocks, G.; Karpan, V. M.; Kelly, P. J.] Univ Twente, MESA Inst Nanotechnol, NL-7500 AE Enschede, Netherlands.
[van den Brink, J.] Radboud Univ Nijmegen, Inst Mol & Mat, Nijmegen, Netherlands.
Reprint Address: Giovannetti, G (reprint author), Leiden Univ, Inst Lorentz Theoret Phys, POB 9506, NL-2300 RA Leiden, Netherlands.
Web of Science Categories: Physics, Multidisciplinary
ISSN: 0031-9007
Title: Hydrogen storage in single-walled carbon nanotubes at room temperature
Author(s): Liu, C (Liu, C); Fan, YY (Fan, YY); Liu, M (Liu, M); Cong, HT (Cong, HT); Cheng, HM (Cheng, HM); Dresselhaus, MS (Dresselhaus, MS)
Source: SCIENCE Volume: 286 Issue: 5442 Pages: 1127-1129 DOI: 10.1126/science.286.5442.1127 Published: NOV 5 1999
Times Cited in Web of Science Core Collection: 1681
Total Times Cited: 1801
Abstract: Masses of single-walled carbon nanotubes (SWNTs) with a large mean diameter of about 1.85 nanometers, synthesized by a semicontinuous hydrogen are discharge method, were employed for hydrogen adsorption experiments in their as-prepared and pretreated states. A hydrogen storage capacity of 4.2 weight percent, or a hydrogen to carbon atom ratio of: 0.52, was achieved reproducibly at room temperature under a modestly high pressure (about 10 megapascal) for a SWNT sample of about 500 milligram weight that was soaked in hydrochloric acid and then heat-treated in vacuum. Moreover, 78.3 percent of the adsorbed hydrogen (3.3 weight percent) could be released under ambient pressure at room temperature, while the release of the residual stored hydrogen (0.9 weight percent) required some heating of the sample. Because the SWNTs can be easily produced and show reproducible and modestly high hydrogen uptake at room temperature, they show promise as an effective hydrogen storage material.
Document Type: Article
Addresses: Chinese Acad Sci, Inst Met Res, Shenyang 110015, Peoples R China.
MIT, Dept Phys, Cambridge, MA 02139 USA.
MIT, Dept Elect Engn & Comp Sci, Cambridge, MA 02139 USA.
Reprint Address: Dresselhaus, MS (reprint author), Chinese Acad Sci, Inst Met Res, 72 Wenhua Rd, Shenyang 110015, Peoples R China.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: ADSORBATE-SUBSTRATE AND ADSORBATE-ADSORBATE INTERACTIONS OF NA AND K ADLAYERS ON AL(111)
Author(s): NEUGEBAUER, J (NEUGEBAUER, J); SCHEFFLER, M (SCHEFFLER, M)
Source: PHYSICAL REVIEW B Volume: 46 Issue: 24 Pages: 16067-16080 DOI: 10.1103/PhysRevB.46.16067 Published: DEC 15 1992
Times Cited in Web of Science Core Collection: 1577
Total Times Cited: 1590
Abstract: We present total-energy, force, and electronic-structure calculations for Na and K adsorbed in various geometries on an Al(111) surface. The calculations apply density-functional theory together with the low-density approximation and the ab initio pseudopotential formalism. Two adsorbate meshes, namely (square-root 3 X square-root 3)R30-degrees and (2 X 2), are considered and for each of them the geometry of the adlayer relative to the substrate is varied over a wide range of possibilities. By total-energy minimization we determine stable and metastable geometries. For Na we find for both adsorbate meshes that the ordering of the calculated binding energies per adatom is such that the substitutional geometry, where each Na atom replaces a surface Al atom, is most favorable and the on-top position is most unfavorable. The (square-root 3 X square-root 3)R30-degrees structure has a lower energy than the (2 X 2) structure. This is shown to be a substrate effect and not an effect of the adsorbate-adsorbate interaction. In contrast to the results for Na, we find for the (square-root 3 X square-root 3)R30-degrees K adsorption that the calculated adsorption energies for the on-top, threefold hollow, and substitutional sites are equal within the accuracy of our calculation, which is +/-0.03 eV. The similarity of the energies of the on-surface adsorption sites is explained as a consequence of the bigger size of K which implies that the adatom experiences a rather small substrate electron-density corrugation. Therefore for potassium the on-top and hollow sites are close in energy already for the unrelaxed Al(111) substrate. Because the relaxation energy of the on-top site is larger than that of the threefold hollow site both sites receive practically the same adsorption energy. The unexpected possibility of surface-substitutional sites is explained as a consequence of the ionic nature of the bonding which, at higher coverages, can develop strongest when the adatom can dive into the substrate as deep as possible. The interesting result of the studied systems is that the difference in bond strengths between the ''normal'' and substitutional geometries is sufficiently large to kick out a surface Al atom.
Document Type: Article
Reprint Address: NEUGEBAUER, J (reprint author), MAX PLANCK GESELL,FRITZ HABER INST,FARADAYWEG 4-6,W-1000 BERLIN 33,GERMANY.
Web of Science Categories: Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 0163-1829
Title: Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H-2 Production Activity of TiO2 Nanoparticles
Author(s): Xiang, QJ (Xiang, Quanjun); Yu, JG (Yu, Jiaguo); Jaroniec, M (Jaroniec, Mietek)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 134 Issue: 15 Pages: 6575-6578 DOI: 10.1021/ja302846n Published: APR 18 2012
Times Cited in Web of Science Core Collection: 1519
Total Times Cited: 1553
Abstract: The production of H-2 by photocatalytic water splitting has attracted a lot attention as a clean and renewable solar H-2 generation system. Despite tremendous efforts, the present great challenge in materials science is to develop highly active photocatalysts for splitting of water at low cost. Here we report a new composite material consisting of TiO2 nanocrystals grown in the presence of a layered MoS2/graphene hybrid as a high-performance photocatalyst for H-2 evolution. This composite material was prepared by a two-step simple hydrothermal process using sodium molybdate, thiourea, and graphene oxide as precursors of the MoS2/graphene hybrid and tetrabutylorthotitanate as the titanium precursor. Even without a noble-metal cocatalyst, the TiO2/MoS2/graphene composite reaches a high H-2 production rate of 165.3 mu mol h(-1) when the content of the MoS2/graphene cocatalyst is 0.5 wt 96 and the content of graphene in this cocatalyst is 5.0 wt 96, and the apparent quantum efficiency reaches 9.796 at 365 nm. This unusual photocatalytic activity arises from the positive synergetic effect between the MoS2 and graphene components in this hybrid cocatalyst, which serve as an electron collector and a source of active adsorption sites, respectively. This study presents an inexpensive photocatalyst for energy conversion to achieve highly efficient H-2 evolution without noble metals.
Document Type: Article
Addresses: [Xiang, Quanjun; Yu, Jiaguo] Wuhan Univ Technol, State Key Lab Adv Technol Mat Synth & Proc, Wuhan 430070, Peoples R China.
[Jaroniec, Mietek] Kent State Univ, Dept Chem, Kent, OH 44242 USA.
Reprint Address: Yu, JG (reprint author), Wuhan Univ Technol, State Key Lab Adv Technol Mat Synth & Proc, 122 Luoshi Rd, Wuhan 430070, Peoples R China.
E-mail Addresses: jiaguoyu@yahoo.com; jaroniec@kent.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: WHY GOLD IS THE NOBLEST OF ALL THE METALS
Author(s): HAMMER, B (HAMMER, B); NORSKOV, JK (NORSKOV, JK)
Source: NATURE Volume: 376 Issue: 6537 Pages: 238-240 DOI: 10.1038/376238a0 Published: JUL 20 1995
Times Cited in Web of Science Core Collection: 1482
Total Times Cited: 1498
Abstract: THE unique role that gold plays in society is to a large extent related to the fact that it is the most noble of all metals: it is the least reactive metal towards atoms or molecules at the interface with a gas or a liquid. The inertness of gold does not reflect a general inability to form chemical bonds, however-gold forms very stable alloys with many other metals. To understand the nobleness of gold, we have studied a simple surface reaction, the dissociation of H-2 On the surface of gold and of three other metals (copper, nickel and platinum) that lie close to it in the periodic table. We present self-consistent density-functional calculations of the activation barriers and chemisorption energies which clearly illustrate that nobleness is related to two factors: the degree of filling of the antibonding states on adsorption, and the degree of orbital overlap with the adsorbate. These two factors, which determine both the strength of the adsorbate-metal interaction and the energy barrier for dissociation, operate together to the maximal detriment of adsorbate binding and subsequent reactivity on gold.
Document Type: Article
Addresses: JOINT RES CTR ATOM TECHNOL,TSUKUBA,IBARAKI 305,JAPAN.
Reprint Address: HAMMER, B (reprint author), TECH UNIV DENMARK,DEPT PHYS,CTR ATOM SCALE MAT PHYS,DK-2800 LYNGBY,DENMARK.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: Periodic mesoporous organosilicas with organic groups inside the channel walls
Author(s): Asefa, T (Asefa, T); MacLachlan, MJ (MacLachlan, MJ); Coombs, N (Coombs, N); Ozin, GA (Ozin, GA)
Source: NATURE Volume: 402 Issue: 6764 Pages: 867-871 Published: DEC 23 1999
Times Cited in Web of Science Core Collection: 1475
Total Times Cited: 1502
Abstract: Surfactant-mediated synthesis methods have attracted much interest for the production of inorganic mesoporous materials, which can, on removal of the surfactant template, incorporate polymeric, organic, inorganic and organometallic 'guests' in their pores(1,2). These materials-initially made of silica(3-5), but now also available in the form of other oxides(6-9), sulphides(10,11), phosphates(12) and metals(13)-could find application in fields ranging from catalysis, adsorption and sensing technology to nanoelectronics. The extension of surfactant-mediated synthesis to produce inorganic-organic hybrid material (that is, materials that contain organic groups as an integral part of their framework structure) promises access to an even wider range of application possibilities. Such hybrid materials have been produced in the form of amorphous silicates (xerogels) that indeed display unique properties different to those of the individual components(14-20), but their random networks with broad pore-size distributions severely limit the shape and size selectivity of these materials. Mesoporous hybrid materials with periodic frameworks have been synthesized, but the organic groups are all terminally bonded to the pore surface, rather than incorporated into the pore walls(21-26). Here we describe a periodic mesoporous organosilica containing bridge-bonded ethene groups directly integrated into the silica framework. We are able to solvent-extract and ion-exchange the surfactant templates to create a stable and periodic mesoporous ethenesilica with high surface area and ethene groups that are readily accessible for chemical reaction. Recent syntheses of similar periodic mesoporous organosilicas(27,28) and the ability to incorporate a variety of bridging organic and organometallic species raise the prospect of being able to fuse organic synthesis and inorganic materials chemistry to generate new materials with interesting chemical, mechanical electronic, optical and magnetic properties.
Document Type: Article
Addresses: Univ Toronto, Dept Chem, Mat Chem Res Grp, Toronto, ON M5S 3H6, Canada.
Reprint Address: Ozin, GA (reprint author), Univ Toronto, Dept Chem, Mat Chem Res Grp, 80 St George St, Toronto, ON M5S 3H6, Canada.
E-mail Addresses: gozin@alchemy.chem.utoronto.ca
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
eISSN: 1476-4687
Title: ADSORPTION OF CHAIN MOLECULES WITH A POLAR HEAD A-SCALING DESCRIPTION
Author(s): ALEXANDER, S (ALEXANDER, S)
Source: JOURNAL DE PHYSIQUE Volume: 38 Issue: 8 Pages: 983-987 DOI: 10.1051/jphys:01977003808098300 Published: 1977
Times Cited in Web of Science Core Collection: 1474
Total Times Cited: 1479
Document Type: Article
Addresses: COLL FRANCE,PHYS MATIERE CONDENSEE LAB,F-75231 PARIS 5,FRANCE.
ISSN: 0302-0738
Title: Chemical accuracy for the van der Waals density functional
Author(s): Klimes, J (Klimes, Jiri); Bowler, DR (Bowler, David R.); Michaelides, A (Michaelides, Angelos)
Source: JOURNAL OF PHYSICS-CONDENSED MATTER Volume: 22 Issue: 2 Article Number: 022201 DOI: 10.1088/0953-8984/22/2/022201 Published: JAN 20 2010
Times Cited in Web of Science Core Collection: 1472
Total Times Cited: 1476
Abstract: The non-local van der Waals density functional (vdW-DF) of Dion et al (2004 Phys. Rev. Lett. 92 246401) is a very promising scheme for the efficient treatment of dispersion bonded systems. We show here that the accuracy of vdW-DF can be dramatically improved both for dispersion and hydrogen bonded complexes through the judicious selection of its underlying exchange functional. New and published exchange functionals are identified that deliver much better than chemical accuracy from vdW-DF for the S22 benchmark set of weakly interacting dimers and for water clusters. Improved performance for the adsorption of water on salt is also obtained.
Document Type: Article
Addresses: [Klimes, Jiri; Bowler, David R.; Michaelides, Angelos] UCL, London Ctr Nanotechnol, London WC1E 6BT, England.
[Klimes, Jiri; Michaelides, Angelos] UCL, Dept Chem, London WC1E 6BT, England.
[Bowler, David R.] UCL, Dept Phys & Astron, London WC1E 6BT, England.
Reprint Address: Klimes, J (reprint author), UCL, London Ctr Nanotechnol, Mortimer St, London WC1E 6BT, England.
E-mail Addresses: angelos.michaelides@ucl.ac.uk
Web of Science Categories: Physics, Condensed Matter
ISSN: 0953-8984
Title: High weight fraction surfactant solubilization of single-wall carbon nanotubes in water
Author(s): Islam, MF (Islam, MF); Rojas, E (Rojas, E); Bergey, DM (Bergey, DM); Johnson, AT (Johnson, AT); Yodh, AG (Yodh, AG)
Source: NANO LETTERS Volume: 3 Issue: 2 Pages: 269-273 DOI: 10.1021/nl025924u Published: FEB 2003
Times Cited in Web of Science Core Collection: 1431
Total Times Cited: 1475
Abstract: We report a simple process to solubilize high weight fraction single-wall carbon nanotubes in water by the nonspecific physical adsorption of sodium dodecylbenzene sulfonate. The diameter distribution of nanotubes in the dispersion, measured by atomic force microscopy, showed that even at 20 mg/mL similar to63 +/- 5% of single-wall carbon nanotube bundles exfoliated into single tubes. A measure of the length distribution of the nanotubes showed that our dispersion technique reduced nanotube fragmentation.
Document Type: Article
Addresses: Univ Penn, Dept Phys & Astron, Philadelphia, PA 19104 USA.
Reprint Address: Islam, MF (reprint author), Univ Penn, Dept Phys & Astron, 209 S 33rd St, Philadelphia, PA 19104 USA.
E-mail Addresses: islam@physics.upenn.edu
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Nanoscience & Nanotechnology; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1530-6984
eISSN: 1530-6992
Title: REVERSIBLE WORK TRANSITION-STATE THEORY - APPLICATION TO DISSOCIATIVE ADSORPTION OF HYDROGEN
Author(s): MILLS, G (MILLS, G); JONSSON, H (JONSSON, H); SCHENTER, GK (SCHENTER, GK)
Source: SURFACE SCIENCE Volume: 324 Issue: 2-3 Pages: 305-337 DOI: 10.1016/0039-6028(94)00731-4 Published: FEB 10 1995
Times Cited in Web of Science Core Collection: 1401
Total Times Cited: 1406
Abstract: A practical method for finding free energy barriers for transitions in high-dimensional classical and quantum systems is presented and used to calculate the dissociative sticking probability of H-2 On a metal surface within the transition state theory, The reversible work involved in shifting the system confined to a hyperplane from the reactant region towards products is evaluated directly. Quantum mechanical degrees of freedom are included by using Feynman path integrals with the hyperplane constraint applied to the centroid of the cyclic paths. An optimal dividing surface for the rate estimated by the transition state theory is identified naturally in the course of the reversible work evaluation. The free energy barrier is determined relative to the reactant state directly so that an estimate of the transition rate can be obtained without requiring a solvable reference model for the transition state. The method has been applied to calculations of the sticking probability of a thermalized hydrogen gas on a Cu(110) surface. The two hydrogen atoms and eight surface Cu atoms were included quantum mechanically and over two hundred atoms in the Cu crystal where included classically. The activation energy for adsorption and desorption was determined and found to be significantly lowered by tunneling at low temperature. The calculated values agree quite well with experimental estimates for adsorption and desorption. Dynamical corrections to the classical transition state theory rate estimate were evaluated and found to be small.
Document Type: Article
Addresses: UNIV WASHINGTON, DEPT CHEM, SEATTLE, WA 98195 USA.
PACIFIC NW LAB, MOLEC SCI RES CTR, RICHLAND, WA 99352 USA.
Web of Science Categories: Chemistry, Physical; Physics, Condensed Matter
ISSN: 0039-6028
Title: Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks
Author(s): Inagaki, S (Inagaki, S); Guan, S (Guan, S); Fukushima, Y (Fukushima, Y); Ohsuna, T (Ohsuna, T); Terasaki, O (Terasaki, O)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 121 Issue: 41 Pages: 9611-9614 DOI: 10.1021/ja9916658 Published: OCT 20 1999
Times Cited in Web of Science Core Collection: 1399
Total Times Cited: 1423
Abstract: Novel organic-inorganic hybrid mesoporous materials have been synthesized, in which organic and inorganic oxide moieties are distributed homogeneously at the molecular level in the framework, forming a covalently bonded network. They are highly ordered at the mesoscale, with two- and three-dimensional hexagonal symmetries and well-defined external morphologies. Nitrogen adsorption measurements show a uniform pore-size distribution with pore diameters of 31 and 27 Angstrom, and high surface areas of 750 and 1170 m(2)/g. The synthetic procedure to polymerize the organosilane monomer containing two trialkoxysilyl groups in the presence of surfactant can be applied to the synthesis of a variety of highly ordered organic-inorganic hybrid mesoporous materials.
Document Type: Article
Addresses: Toyota Cent Res & Dev Labs Inc, Nagakute, Aichi 4801192, Japan.
Tohoku Univ, Inst Mat Res, Sendai, Miyagi 9808577, Japan.
Tohoku Univ, JST, CREST, Sendai, Miyagi 9808578, Japan.
Tohoku Univ, Grad Sch Sci, Dept Phys, Sendai, Miyagi 9808578, Japan.
Reprint Address: Inagaki, S (reprint author), Toyota Cent Res & Dev Labs Inc, 41-1 Aza Yokomichi, Nagakute, Aichi 4801192, Japan.
E-mail Addresses: inagaki@mosk.tytlabs.co.jp
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: HIGH-PERFORMANCE ELECTROPHORESIS - ELIMINATION OF ELECTROENDOSMOSIS AND SOLUTE ADSORPTION
Author(s): HJERTEN, S (HJERTEN, S)
Source: JOURNAL OF CHROMATOGRAPHY Volume: 347 Issue: 2 Pages: 191-198 DOI: 10.1016/S0021-9673(01)95485-8 Published: 1985
Times Cited in Web of Science Core Collection: 1395
Total Times Cited: 1411
Document Type: Article
Reprint Address: HJERTEN, S (reprint author), UNIV UPPSALA,CTR BIOMED,INST BIOCHEM,POB 576,S-75123 UPPSALA,SWEDEN.
Web of Science Categories: Chemistry, Analytical
ISSN: 0021-9673
Title: THE POTENTIAL THEORY OF ADSORPTION OF GASES AND VAPORS FOR ADSORBENTS WITH ENERGETICALLY NONUNIFORM SURFACES
Author(s): DUBININ, MM (DUBININ, MM)
Source: CHEMICAL REVIEWS Volume: 60 Issue: 2 Pages: 235-241 DOI: 10.1021/cr60204a006 Published: 1960
Times Cited in Web of Science Core Collection: 1388
Total Times Cited: 1439
Document Type: Article
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0009-2665
Title: Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts
Author(s): Strasser, P (Strasser, Peter); Koh, S (Koh, Shirlaine); Anniyev, T (Anniyev, Toyli); Greeley, J (Greeley, Jeff); More, K (More, Karren); Yu, CF (Yu, Chengfei); Liu, ZC (Liu, Zengcai); Kaya, S (Kaya, Sarp); Nordlund, D (Nordlund, Dennis); Ogasawara, H (Ogasawara, Hirohito); Toney, MF (Toney, Michael F.); Nilsson, A (Nilsson, Anders)
Source: NATURE CHEMISTRY Volume: 2 Issue: 6 Pages: 454-460 DOI: 10.1038/NCHEM.623 Published: JUN 2010
Times Cited in Web of Science Core Collection: 1387
Total Times Cited: 1399
Abstract: Electrocatalysis will play a key role in future energy conversion and storage technologies, such as water electrolysers, fuel cells and metal-air batteries. Molecular interactions between chemical reactants and the catalytic surface control the activity and efficiency, and hence need to be optimized; however, generalized experimental strategies to do so are scarce. Here we show how lattice strain can be used experimentally to tune the catalytic activity of dealloyed bimetallic nanoparticles for the oxygen-reduction reaction, a key barrier to the application of fuel cells and metal-air batteries. We demonstrate the core-shell structure of the catalyst and clarify the mechanistic origin of its activity. The platinum-rich shell exhibits compressive strain, which results in a shift of the electronic band structure of platinum and weakening chemisorption of oxygenated species. We combine synthesis, measurements and an understanding of strain from theory to generate a reactivity-strain relationship that provides guidelines for tuning electrocatalytic activity.
Document Type: Article
Addresses: [Strasser, Peter] Tech Univ Berlin, Div Chem Engn, Dept Chem, Electrochem Energy Catalysis & Mat Sci Lab, D-10623 Berlin, Germany.
[Strasser, Peter; Koh, Shirlaine; Yu, Chengfei; Liu, Zengcai] Univ Houston, Dept Chem & Biomol Engn, Houston, TX 77204 USA.
[Anniyev, Toyli; Kaya, Sarp; Ogasawara, Hirohito; Toney, Michael F.; Nilsson, Anders] Stanford Inst Mat & Energy Sci, SLAC Natl Accelerator Lab, Menlo Pk, CA 94025 USA.
[Anniyev, Toyli; Kaya, Sarp; Nordlund, Dennis; Ogasawara, Hirohito; Toney, Michael F.; Nilsson, Anders] SLAC Natl Accelerator Lab, Stanford Synchrotron Radiat Lightsource, Menlo Pk, CA 94025 USA.
[Greeley, Jeff] Argonne Natl Lab, Ctr Nanoscale Mat, Argonne, IL 60439 USA.
[More, Karren] Oak Ridge Natl Lab, Div Mat Sci & Technol, Oak Ridge, TN 37831 USA.
Reprint Address: Strasser, P (reprint author), Tech Univ Berlin, Div Chem Engn, Dept Chem, Electrochem Energy Catalysis & Mat Sci Lab, D-10623 Berlin, Germany.
E-mail Addresses: pstrasser@tu-berlin.de
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 1755-4330
eISSN: 1755-4349
Title: An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel
Author(s): Song, CS (Song, CS)
Source: CATALYSIS TODAY Volume: 86 Issue: 1-4 Pages: 211-263 DOI: 10.1016/S0920-5861(03)00412-7 Published: NOV 1 2003
Times Cited in Web of Science Core Collection: 1387
Total Times Cited: 1605
Abstract: This review discusses the problems of sulfur reduction in highway and non-road fuels and presents an overview of new approaches and emerging technologies for ultra-deep desulfurization of refinery streams for ultra-clean (ultra-low-sulfur) gasoline, diesel fuels and jet fuels. The issues of gasoline and diesel deep desulfurization are becoming more serious because the crude oils refined in the US are getting higher in sulfur contents and heavier in density, while the regulated sulfur limits are becoming lower and lower. Current gasoline desulfurization problem is dominated by the issues of sulfur removal from FCC naphtha, which contributes about 35% of gasoline pool but over 90% of sulfur in gasoline. Deep reduction of gasoline sulfur (from 330 to 30 ppm) must be made without decreasing octane number or losing gasoline yield. The problem is complicated by the high olefins contents of FCC naphtha which contributes to octane number enhancement but can be saturated under HDS conditions. Deep reduction of diesel sulfur (from 500 to < 15 ppm sulfur) is dictated largely by 4,6-dimethyldibenzothiophene, which represents the least reactive sulfur compounds that have substitutions on both 4- and 6-positions. The deep HDS problem of diesel streams is exacerbated by the inhibiting effects of co-existing polyaromatics and nitrogen compounds in the feed as well as H(2)S in the product. The approaches to deep desulfurization include catalysts and process developments for hydrodesulfurization (HDS), and adsorbents or reagents and methods for non-HDS-type processing schemes. The needs for dearomatization of diesel and jet fuels are also discussed along with some approaches. Overall, new and more effective approaches and continuing catalysis and processing research are needed for producing affordable ultra-clean (ultra-low-sulfur and low-aromatics) transportation fuels and non-road fuels, because meeting the new government sulfur regulations in 2006-2010 (15 ppm sulfur in highway diesel fuels by 2006 and non-road diesel fuels by 20 10; 30 ppm sulfur in gasoline by 2006) is only a milestone. Desulfurization research should also take into consideration of the fuel-cell fuel processing needs, which will have a more stringent requirement on desulfurization (e.g., < 1 ppm sulfur) than IC engines. The society at large is stepping on the road to zero sulfur fuel, so researchers should begin with the end in mind and try to develop long-term solutions. (C) 2003 Elsevier B.V. All rights reserved.
Document Type: Article; Proceedings Paper
Conference Title: International Symposium on Ultra-Clean Transportation Fuels held at the National Meeting of the American-Chemical-Society
Conference Date: AUG 18-22, 2002
Conference Location: BOSTON, MA
Addresses: Penn State Univ, Energy Inst, Dept Energy & Geoenvironm Engn, Clean Fuels & Catalysis Program, University Pk, PA 16802 USA.
Reprint Address: Song, CS (reprint author), Penn State Univ, Energy Inst, Dept Energy & Geoenvironm Engn, Clean Fuels & Catalysis Program, University Pk, PA 16802 USA.
E-mail Addresses: csong@psu.edu
Web of Science Categories: Chemistry, Applied; Chemistry, Physical; Engineering, Chemical
ISSN: 0920-5861
Title: ORGANIZED MONOLAYERS BY ADSORPTION .1. FORMATION AND STRUCTURE OF OLEOPHOBIC MIXED MONOLAYERS ON SOLID-SURFACES
Author(s): SAGIV, J (SAGIV, J)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 102 Issue: 1 Pages: 92-98 DOI: 10.1021/ja00521a016 Published: 1980
Times Cited in Web of Science Core Collection: 1372
Total Times Cited: 1405
Document Type: Article
Reprint Address: SAGIV, J (reprint author), MAX PLANCK INST BIOPHYS CHEM, KARL FRIEDRICH BONHOEFFER INST, MOLEK SYST AUFBAU ABT, D-3400 GOTTINGEN, FED REP GER.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Graphene-Wrapped Fe3O4 Anode Material with Improved Reversible Capacity and Cyclic Stability for Lithium Ion Batteries
Author(s): Zhou, GM (Zhou, Guangmin); Wang, DW (Wang, Da-Wei); Li, F (Li, Feng); Zhang, LL (Zhang, Lili); Li, N (Li, Na); Wu, ZS (Wu, Zhong-Shuai); Wen, L (Wen, Lei); Lu, GQ (Lu, Gao Qing (Max)); Cheng, HM (Cheng, Hui-Ming)
Source: CHEMISTRY OF MATERIALS Volume: 22 Issue: 18 Pages: 5306-5313 DOI: 10.1021/cm101532x Published: SEP 28 2010
Times Cited in Web of Science Core Collection: 1366
Total Times Cited: 1394
Abstract: A well-organized flexible interleaved composite of graphene nanosheets (GNSs) decorated with Fe3O4 particles was synthesized through in situ reduction of iron hydroxide between GNSs. The GNS/Fe3O4 composite shows a reversible specific capacity approaching 1026 mA h(-1) g(-1) after 30 cycles at 35 mA g(-1) 580 mAh g(-1) after 100 cycles at 700 mA g(-1) as well as improved cyclic stability and excellent rate capability. The multifunctional features of the GNS/Fe3O4 composite are considered as follows: (i) GNSs play a "flexible confinement" function to enwrap Fe3O4 particles, which can compensate for the volume change of Fe3O4 and prevent the detachment and agglomeration of pulverized Fe3O4, thus extending the cycling life of the electrode; (ii) GNSs provide a large contact surface for individual dispersion of well-adhered Fe3O4 particles and act as an excellent conductive agent to provide a highway for electron transport, improving the accessible capacity; (iii) Fe3O4 particles separate GNSs and prevent their restacking thus improving the adsorption and immersion of electrolyte on the surface of electroactive material; and (iv) the porosity formed by lateral GNSs and Fe3O4 particles facilitates ion transportation. As a result, this unique laterally confined GNS/Fe3O4 composite can dramatically improve the cycling stability and the rate capability of Fe3O4 as an anode material for lithium ion batteries.
Document Type: Article
Addresses: [Zhou, Guangmin; Li, Feng; Zhang, Lili; Li, Na; Wu, Zhong-Shuai; Wen, Lei; Cheng, Hui-Ming] Chinese Acad Sci, Inst Met Res, Shenyang Natl Lab Mat Sci, Shenyang 110016, Peoples R China.
[Wang, Da-Wei; Lu, Gao Qing (Max)] Univ Queensland, AIBN, ARC Ctr Excellence Funct Nanomat, Brisbane, Qld 4072, Australia.
Reprint Address: Li, F (reprint author), Chinese Acad Sci, Inst Met Res, Shenyang Natl Lab Mat Sci, 72 Wenhua Rd, Shenyang 110016, Peoples R China.
E-mail Addresses: fli@imr.ac.cn; cheng@imr.ac.cn
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0897-4756
eISSN: 1520-5002
Title: ADSORPTION, AUTOINHIBITION AND AUTOCATALYSIS IN POLAROGRAPHY AND IN LINEAR POTENTIAL SWEEP VOLTAMMETRY
Author(s): LAVIRON, E (LAVIRON, E)
Source: JOURNAL OF ELECTROANALYTICAL CHEMISTRY Volume: 52 Issue: 3 Pages: 355-393 DOI: 10.1016/S0022-0728(74)80448-1 Published: 1974
Times Cited in Web of Science Core Collection: 1337
Total Times Cited: 1411
Document Type: Article
Addresses: FAC SCI GABRIEL,CNRS,LAB POLAROG ORG,6 BLVD GABRIEL,21000 DIJON,FRANCE.
Web of Science Categories: Chemistry, Analytical; Electrochemistry
ISSN: 0022-0728
Title: A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules
Author(s): Lai, CY (Lai, CY); Trewyn, BG (Trewyn, BG); Jeftinija, DM (Jeftinija, DM); Jeftinija, K (Jeftinija, K); Xu, S (Xu, S); Jeftinija, S (Jeftinija, S); Lin, VSY (Lin, VSY)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 125 Issue: 15 Pages: 4451-4459 DOI: 10.1021/ja028650l Published: APR 16 2003
Times Cited in Web of Science Core Collection: 1331
Total Times Cited: 1363
Abstract: An MCM-41 type mesoporous silica nanosphere-based (MSN) control led- release delivery system has been synthesized and characterized using surface-derivatized cadmium sulfide (CdS) nanocrystals as chemically removable caps to encapsulate several pharmaceutical drug molecules and neurotransmitters; inside the organically functionalized MSN mesoporous framework. We studied the stimuli-responsive release profiles of vancomycin- and adenosine triphosphate (ATP)-loaded MSN delivery systems by using disulfide bond-reducing molecules, such as dithiothreitol (DTT) and mercaptoethanol (ME), as release triggers. The biocompatibility and delivery efficiency of the MSN system with neuroglial cells (astrocytes) in vitro were demonstrated. In contrast to many current delivery systems, the molecules of interest were encapsulated inside the porous framework of the MSN not by adsorption or sol-gel types of entrapment but by capping the openings of the mesoporous channels with size-defined CdS nanoparticles to physically block the drugs/ neurotransmitters of certain sizes from leaching out. We envision that this new MSN system could play a significant role in developing new generations of site-selective, control led-release delivery nanodevices.
Document Type: Article
Addresses: Iowa State Univ, Dept Chem, Ames, IA 50011 USA.
Iowa State Univ, Dept Biomed Sci, Ames, IA 50011 USA.
Reprint Address: Lin, VSY (reprint author), Iowa State Univ, Dept Chem, Ames, IA 50011 USA.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: STATISTICAL-THEORY OF THE ADSORPTION OF INTERACTING CHAIN MOLECULES .1. PARTITION-FUNCTION, SEGMENT DENSITY DISTRIBUTION, AND ADSORPTION-ISOTHERMS
Author(s): SCHEUTJENS, JMHM (SCHEUTJENS, JMHM); FLEER, GJ (FLEER, GJ)
Source: JOURNAL OF PHYSICAL CHEMISTRY Volume: 83 Issue: 12 Pages: 1619-1635 DOI: 10.1021/j100475a012 Published: 1979
Times Cited in Web of Science Core Collection: 1327
Total Times Cited: 1337
Document Type: Article
Reprint Address: SCHEUTJENS, JMHM (reprint author), LAB PHYS & COLLOID CHEM,DE DREIJEN 6,WAGENINGEN,NETHERLANDS.
Web of Science Categories: Chemistry, Physical
ISSN: 0022-3654
Title: Emulsions stabilised solely by colloidal particles
Author(s): Aveyard, R (Aveyard, R); Binks, BP (Binks, BP); Clint, JH (Clint, JH)
Source: ADVANCES IN COLLOID AND INTERFACE SCIENCE Volume: 100 Special Issue: SI Pages: 503-546 Article Number: PII S0001-8686(02)00069-6 DOI: 10.1016/S0001-8686(02)00069-6 Published: FEB 28 2003
Times Cited in Web of Science Core Collection: 1321
Total Times Cited: 1366
Abstract: The preparation and properties of emulsions, stabilised solely by the adsorption of solid particles at the oil-water interface, are reviewed especially in the light of our own work with particles of well-controlled surface properties. Where appropriate, comparison is made with the behaviour of surfactant-stabilised emulsions. Hydrophilic particles tend to form oil-in-water (o/w) emulsions whereas hydrophobic particles form water-in-oil (w/o) emulsions. Many of the properties can be attributed to the very large free energy of adsorption for particles of intermediate wettability (contact angle at the oil-water interface, say, between 50 and 130degrees). This effectively irreversible adsorption leads to extreme stability for certain emulsions and is in contrast to the behaviour of surfactant molecules which are usually in rapid dynamic equilibrium between the oil-water interface and the bulk phases. There is. evidence that, in some systems, weak flocculation of the particles improves the emulsion stability. Phase inversion from w/o to o/w can be brought about by increasing the volume fraction of water. Emulsions close to this inversion point tend to be the most stable, again in contrast to surfactant systems. The volume fraction needed for inversion depends on the particle wettability and the nature of the oil and these effects have been rationalised in terms of surface energy components. Stable multiple emulsions (w/o/w and o/w/o) can be made using two types of particles with slightly different wettability. Similar multiple emulsions prepared with two types of surfactant tend to be much less stable. The possibility of preparing novel solid materials by evaporating solid-stabilised emulsions is also proposed. Finally we report on some extensions to the work of Levine et al. who obtained expressions for the free energy of formation of emulsion drops covered with close-packed monolayers of monodisperse spherical particles. In particular in the light of the observations that nanoparticles can act as excellent emulsion stabilisers, we have considered potential effects on the free energy of emulsion formation of the action of small (physically realistic) positive and negative line tensions in the 3-phase contact lines skirting particles adsorbed at the droplet interfaces. We also explore the possibility that curvature properties of close-packed particle monolayers can affect emulsion properties in much the same way that surfactant monolayer properties influence emulsion type and stability. (C) 2002 Elsevier Science B.V. All rights reserved.
Document Type: Article
Addresses: Univ Hull, Dept Chem, Surface & Colloid Grp, Kingston Upon Hull HU6 7RX, N Humberside, England.
Reprint Address: Binks, BP (reprint author), Univ Hull, Dept Chem, Surface & Colloid Grp, Kingston Upon Hull HU6 7RX, N Humberside, England.
Web of Science Categories: Chemistry, Physical
ISSN: 0001-8686
Title: Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks
Author(s): Rowsell, JLC (Rowsell, JLC); Yaghi, OM (Yaghi, OM)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 128 Issue: 4 Pages: 1304-1315 DOI: 10.1021/ja056639q Published: FEB 1 2006
Times Cited in Web of Science Core Collection: 1319
Total Times Cited: 1337
Abstract: The dihydrogen adsorption isotherms of eight metal-organic frameworks (MOFs), measured at 77 K up to a pressure of 1 atm, have been examined for correlations with their structural features. All materials display approximately Type I isotherms with no hysteresis, and saturation was not reached for any of the materials under these conditions. Among the six isoreticular MOFs (IRMOFs) studied, the catenated materials exhibit the largest capacities on a molar basis, up to 9.8 H-2 per formula unit. The addition of functional groups (-Br, -NH2, -C2H4-) to the phenylene links of IRMOF-1 (MOF-5), or their replacement with thieno[3,2-b]thiophene moieties in IRMOF-20, altered the adsorption behavior by a minor amount despite large variations in the pore volumes of the resulting materials. In contrast, replacement of the metal oxide units with those containing coordinatively unsaturated metal sites resulted in greater H2 uptake. The enhanced affinities of these materials, MOF-74 and HKUST-1, were further demonstrated by calculation of the isosteric heats of adsorption, which were larger across much of the range of coverage examined, compared to those of representative IRMOFs. The results suggest that under low-loading conditions, the H2 adsorption behavior of MOFs can be improved by imparting larger charge gradients on the metal oxide units and adjusting the link metrics to constrict the pore dimensions; however, a large pore volume is still a prerequisite feature.
Document Type: Article
Addresses: Univ Michigan, Dept Chem, Ann Arbor, MI 48109 USA.
Reprint Address: Yaghi, OM (reprint author), Univ Michigan, Dept Chem, 930 N Univ Ave, Ann Arbor, MI 48109 USA.
E-mail Addresses: oyaghi@umich.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: SELECTIVE BINDING AND REMOVAL OF GUESTS IN A MICROPOROUS METAL-ORGANIC FRAMEWORK
Author(s): YAGHI, OM (YAGHI, OM); LI, GM (LI, GM); LI, HL (LI, HL)
Source: NATURE Volume: 378 Issue: 6558 Pages: 703-706 DOI: 10.1038/378703a0 Published: DEC 14 1995
Times Cited in Web of Science Core Collection: 1313
Total Times Cited: 1343
Abstract: MICROPOROUS inorganic materials such as zeolites find widespread application in heterogeneous catalysis, adsorption and ion-exchange processes. The rigidity and stability of such frameworks allow for shape- and size-selective inclusion of organic molecules and ions(1-4). Analogous microporous structures based on organic building blocks have the potential for more precise rational design, through control of the shape, size and functionalization of the pores(5-8). Here we report the synthesis of a metal-organic framework designed to bind aromatic guest molecules selectively. The basic building block is a symmetric organic molecule, which binds metal ions(9,10) to form layers of the metal-organic compound alternating with layers whose composition is determined by the functionalization of the starting molecules. The layers create channels in which guest aromatic molecules may be selectively bound, We show that the crystal lattice thus formed is thermally stable up to 350 degrees C, even after removal of included guest molecules, and that the inclusions can be selectively readsorbed.
Document Type: Article
Reprint Address: YAGHI, OM (reprint author), ARIZONA STATE UNIV,GOLDWATER CTR SCI & ENGN,DEPT CHEM & BIOCHEM,TEMPE,AZ 85287, USA.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: Continuous formation of supported cubic and hexagonal mesoporous films by sol gel dip-coating
Author(s): Lu, YF (Lu, YF); Ganguli, R (Ganguli, R); Drewien, CA (Drewien, CA); Anderson, MT (Anderson, MT); Brinker, CJ (Brinker, CJ); Gong, WL (Gong, WL); Guo, YX (Guo, YX); Soyez, H (Soyez, H); Dunn, B (Dunn, B); Huang, MH (Huang, MH); Zink, JI (Zink, JI)
Source: NATURE Volume: 389 Issue: 6649 Pages: 364-368 DOI: 10.1038/38699 Published: SEP 25 1997
Times Cited in Web of Science Core Collection: 1312
Total Times Cited: 1348
Abstract: Thin films of surfactant-templated mesoporous materials(1,2) could find applications in membrane-based separations, selective catalysis and sensors. Above the critical micelle concentration of a bulk silica-surfactant solution, films of mesophases with hexagonally packed one-dimensional channels can be formed at solid-liquid and liquid-vapour interfaces(3-5). But this process is slow and the supported films(3,5) are granular and with the pore channels oriented parallel to the substrate surface, so that transport across the films is not facilitated by the pores. Ogawa(6,7) has reported a rapid spin-coating procedure for making transparent mesoporous films, but their formation mechanism, microstructure and pore accessibility have not been elucidated. Here we report a sol-gel-based dip-coating method for the rapid synthesis of continuous mesoporous thin films on a solid substrate. The influence of the substrate generates film mesostructures that have no bulk counterparts, such as composites with incipient liquid-crystalline order of the surfactant-silica phase. We are also able to form mesoporous films of the cubic phase, in which the pores are connected in a three-dimensional network that guarantees their accessibility from the film surface. We demonstrate and quantify this accessibility using a surface-acoustic-wave nitrogen-adsorption technique. We use fluorescence depolarization to monitor the evolution of the mesophase in situ, and see a progression through a sequence of lamellar to cubic to hexagonal structures that has not previously been reported.
Document Type: Article
Addresses: SANDIA NATL LABS, ALBUQUERQUE, NM 87106 USA.
UNIV NEW MEXICO, NSF, CTR MICROENGINEERED MAT, ALBUQUERQUE, NM 87106 USA.
UNIV NEW MEXICO, DEPT EARTH & PLANETARY SCI, ALBUQUERQUE, NM 87106 USA.
UNIV CALIF LOS ANGELES, DEPT MAT SCI, LOS ANGELES, CA 90095 USA.
UNIV CALIF LOS ANGELES, DEPT CHEM, LOS ANGELES, CA 90095 USA.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: ADSORPTION OF PROTEINS ONTO SURFACES CONTAINING END-ATTACHED OLIGO(ETHYLENE OXIDE) - A MODEL SYSTEM USING SELF-ASSEMBLED MONOLAYERS
Author(s): PRIME, KL (PRIME, KL); WHITESIDES, GM (WHITESIDES, GM)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 115 Issue: 23 Pages: 10714-10721 DOI: 10.1021/ja00076a032 Published: NOV 17 1993
Times Cited in Web of Science Core Collection: 1308
Total Times Cited: 1321
Abstract: This paper reports a study of the adsorption of four proteins-fibrinogen, lysozyme, pyruvate kinase, and RNAse A-to self-assembled monolayers (SAMs) on gold. The SAMs examined were derived from thiols of the structure HS(CH2)10R, where R was CH3, CH2OH, and oligo(ethylene oxide). Monolayers that contained a sufficiently large mole fraction of alkanethiolate groups terminated in oligo(ethylene oxide) chains resisted the kinetically irreversible, nonspecific adsorption of all four proteins. Longer chains of oligo(ethylene oxide) were resistant at lower mole fractions in the monolayer. Resistance to the adsorption of proteins increased with the length of the oligo(ethylene oxide) chain: the smallest mole fraction of chains that prevented adsorption was proportional to n-0.4, where n represents the number of ethylene oxide units per chain. Termination of the oligo(ethylene oxide) chains with a methoxy group instead of a hydroxyl group had little or no effect on the amount of protein adsorbed. The amount of pyruvate kinase that adsorbed to mixed SAMs containing hexa(ethylene oxide)-terminated chains depended upon the temperature. When the mole fraction of oligo(ethylene oxide) groups in the monolayer was below the level needed to prevent adsorption, more pyruvate kinase adsorbed to the monolayer at 37-degrees-C than at 25-degrees-C. No difference was observed between adsorption at 25 and 4-degrees-C.
Document Type: Article
Addresses: HARVARD UNIV,DEPT CHEM,CAMBRIDGE,MA 02138.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes
Author(s): Shiratori, SS (Shiratori, SS); Rubner, MF (Rubner, MF)
Source: MACROMOLECULES Volume: 33 Issue: 11 Pages: 4213-4219 DOI: 10.1021/ma991645q Published: MAY 30 2000
Times Cited in Web of Science Core Collection: 1307
Total Times Cited: 1318
Abstract: A detailed study of the role that solution pH plays in the layer-by-layer processing of the weak polyelectrolytes poly(acrylic acid) and poly(allylamine hydrochoride) was carried out. It was found that dramatically different polymer adsorption behavior is observed as one systematically increases (or decreases) the charge density of a weak polyelectrolyte including transitions from very thick adsorbed layers (ca. 80 Angstrom) to very thin adsorbed layers (ca. 4 Angstrom) over a very narrow pH range. By controlling pH, it is possible to vary the thickness of an adsorbed polycation or polyanion layer from 5 to 80 Angstrom. In addition, control over the bulk and surface composition of the resultant multilayer thin films is readily achieved via simple pH adjustments. These studies have provided new insights into the polyelectrolyte sequential adsorption process and have already opened up some interesting technological applications.
Document Type: Article
Addresses: MIT, Dept Mat Sci & Engn, Cambridge, MA 02139 USA.
Keio Univ, Dept Appl Phys & Physicoinformat, Yokohama, Kanagawa 223, Japan.
Reprint Address: Rubner, MF (reprint author), MIT, Dept Mat Sci & Engn, Cambridge, MA 02139 USA.
Web of Science Categories: Polymer Science
ISSN: 0024-9297
Title: BUILDUP OF ULTRATHIN MULTILAYER FILMS BY A SELF-ASSEMBLY PROCESS .1. CONSECUTIVE ADSORPTION OF ANIONIC AND CATIONIC BIPOLAR AMPHIPHILES ON CHARGED SURFACES
Author(s): DECHER, G (DECHER, G); HONG, JD (HONG, JD)
Source: MAKROMOLEKULARE CHEMIE-MACROMOLECULAR SYMPOSIA Volume: 46 Pages: 321-327 DOI: 10.1002/masy.19910460145 Published: JUN 1991
Times Cited in Web of Science Core Collection: 1306
Total Times Cited: 1390
Abstract: An anionic and a cationic bipolar amphiphile containing rigid biphenyl cores were synthesized. The compounds were dissolved in a mixture of dimethylsulfoxide (DMSO) and water and pure water, respectively. When a solid substrate with a positively charged planar surface is immersed in the solution containing the negatively charged bipolar amphiphile, a monolayer of the amphiphile is adsorbed and due to its bipolar structure the surface charge is reversed. After rinsing in pure water the substrate is immersed in the solution containing the positively charged bipolar amphiphile. Again a monolayer is adsorbed but now the original surface charge is restored. By repeating both steps in a cyclic fashion alternating multilayer assemblies of both compounds are obtained. It is demonstrated that multilayer films, composed of at least 35 consecutively alternating layers, which corresponds to a total film thickness of 170 nm can be assembled.
Document Type: Article; Proceedings Paper
Conference Title: 3RD EUROPEAN CONF ON ORGANIZED ORGANIC THIN FILMS ( ECOF 90 )
Conference Date: OCT 11-13, 1990
Conference Location: JOHANNES GUTENBERG UNIV, MAINZ, FED REP GER
Conference Host: JOHANNES GUTENBERG UNIV
Reprint Address: DECHER, G (reprint author), UNIV MAINZ,INST PHYS CHEM,WELDER WEG 11,W-6500 MAINZ,GERMANY.
Web of Science Categories: Polymer Science
ISSN: 0258-0322
Title: Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility
Author(s): Dixit, S (Dixit, S); Hering, JG (Hering, JG)
Source: ENVIRONMENTAL SCIENCE & TECHNOLOGY Volume: 37 Issue: 18 Pages: 4182-4189 DOI: 10.1021/es030309t Published: SEP 15 2003
Times Cited in Web of Science Core Collection: 1299
Total Times Cited: 1346
Abstract: Arsenic derived from natural sources occurs in groundwater in many countries, affecting the health of millions of people. The combined effects of As(V) reduction and diagenesis of iron oxide minerals on arsenic mobility are investigated in this study by comparing As(V) and As(III) sorption onto amorphous iron oxide (HFO), goethite, and magnetite at varying solution compositions. Experimental data are modeled with a diffuse double layer surface complexation model, and the extracted model parameters are used to examine the consistency of our results with those previously reported. Sorption of As(V) onto HFO and goethite is more favorable than that of As(III) below pH 5-6, whereas, above pH 7-8, As(III) has a higher affinity for the solids. The pH at which As(V) and As(III) are equally sorbed depends on the solid-to-solution ratio and type and specific surface area of the minerals and is shifted to lower pH values in the presence of phosphate, which competes for sorption sites. The sorption data indicate that, under most of the chemical conditions investigated in this study, reduction of As(V) in the presence of HFO or goethite would have only minor effects on or even decrease its mobility in the environment at near-neutral pH conditions. As(V) and As(III) sorption isotherms indicate similar surface site densities on the three oxides. Intrinsic surface complexation constants for As(V) are higher for goethite than HFO, whereas As(III) binding is similar for both of these oxides and also for magnetite. However, decrease in specific surface area and hence sorption site density that accompanies transformation of amorphous iron oxides to more crystalline phases could increase arsenic mobility.
Document Type: Article
Addresses: CALTECH, Pasadena, CA 91125 USA.
Reprint Address: Hering, JG (reprint author), CALTECH, 1200 E Calif Blvd,Environm Sci & Engn 138-78, Pasadena, CA 91125 USA.
Web of Science Categories: Engineering, Environmental; Environmental Sciences
ISSN: 0013-936X
Title: Effect of strain on the reactivity of metal surfaces
Author(s): Mavrikakis, M (Mavrikakis, M); Hammer, B (Hammer, B); Norskov, JK (Norskov, JK)
Source: PHYSICAL REVIEW LETTERS Volume: 81 Issue: 13 Pages: 2819-2822 DOI: 10.1103/PhysRevLett.81.2819 Published: SEP 28 1998
Times Cited in Web of Science Core Collection: 1296
Total Times Cited: 1305
Abstract: Self-consistent density functional calculations for the adsorption of O and CO, and the dissociation of CO on strained and unstrained Ru(0001) surfaces are used to show how strained metal surfaces have chemical properties that are significantly different from those of unstrained surfaces. Surface reactivity increases with lattice expansion, following a concurrent up-shift of the metal d states. Consequences for the catalytic activity of thin metal overlayers are discussed.
Document Type: Article
Addresses: Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Phys, DK-2800 Lyngby, Denmark.
Aalborg Univ, Inst Phys, DK-9220 Aalborg, Denmark.
Reprint Address: Mavrikakis, M (reprint author), Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Phys, DK-2800 Lyngby, Denmark.
Web of Science Categories: Physics, Multidisciplinary
ISSN: 0031-9007
eISSN: 1079-7114
Title: Single-atom catalysis of CO oxidation using Pt-1/FeOx
Author(s): Qiao, BT (Qiao, Botao); Wang, AQ (Wang, Aiqin); Yang, XF (Yang, Xiaofeng); Allard, LF (Allard, Lawrence F.); Jiang, Z (Jiang, Zheng); Cui, YT (Cui, Yitao); Liu, JY (Liu, Jingyue); Li, J (Li, Jun); Zhang, T (Zhang, Tao)
Source: NATURE CHEMISTRY Volume: 3 Issue: 8 Pages: 634-641 DOI: 10.1038/NCHEM.1095 Published: AUG 2011
Times Cited in Web of Science Core Collection: 1284
Total Times Cited: 1300
Abstract: Platinum-based heterogeneous catalysts are critical to many important commercial chemical processes, but their efficiency is extremely low on a per metal atom basis, because only the surface active-site atoms are used. Catalysts with single-atom dispersions are thus highly desirable to maximize atom efficiency, but making them is challenging. Here we report the synthesis of a single-atom catalyst that consists of only isolated single Pt atoms anchored to the surfaces of iron oxide nanocrystallites. This single-atom catalyst has extremely high atom efficiency and shows excellent stability and high activity for both CO oxidation and preferential oxidation of CO in H-2. Density functional theory calculations show that the high catalytic activity correlates with the partially vacant 5d orbitals of the positively charged, high-valent Pt atoms, which help to reduce both the CO adsorption energy and the activation barriers for CO oxidation.
Document Type: Article
Addresses: [Qiao, Botao; Wang, Aiqin; Liu, Jingyue; Zhang, Tao] Chinese Acad Sci, Dalian Inst Chem Phys, State Key Lab Catalysis, Dalian 116023, Peoples R China.
[Yang, Xiaofeng; Li, Jun] Tsinghua Univ, Dept Chem, Beijing 100084, Peoples R China.
[Allard, Lawrence F.] Oak Ridge Natl Lab, Div Mat Sci & Technol, Oak Ridge, TN 37831 USA.
[Jiang, Zheng] Chinese Acad Sci, Shanghai Inst Appl Phys, Shanghai Synchrotron Radiat Facil, Shanghai 201204, Peoples R China.
[Cui, Yitao] Chinese Acad Sci, Dalian Inst Chem Phys, State Key Lab Mol React Dynam, Dalian 116023, Peoples R China.
[Liu, Jingyue] Univ Missouri, Dept Phys & Astron, Ctr Nanosci, St Louis, MO 63121 USA.
[Liu, Jingyue] Univ Missouri, Dept Chem & Biochem, St Louis, MO 63121 USA.
Reprint Address: Qiao, BT (reprint author), Chinese Acad Sci, Dalian Inst Chem Phys, State Key Lab Catalysis, Dalian 116023, Peoples R China.
E-mail Addresses: liuj@umsl.edu; junli@tsinghua.edu.cn; taozhang@dicp.ac.cn
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 1755-4330
Title: ASSEMBLY OF MULTICOMPONENT PROTEIN FILMS BY MEANS OF ELECTROSTATIC LAYER-BY-LAYER ADSORPTION
Author(s): LVOV, Y (LVOV, Y); ARIGA, K (ARIGA, K); ICHINOSE, I (ICHINOSE, I); KUNITAKE, T (KUNITAKE, T)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 117 Issue: 22 Pages: 6117-6123 DOI: 10.1021/ja00127a026 Published: JUN 7 1995
Times Cited in Web of Science Core Collection: 1273
Total Times Cited: 1302
Abstract: Multilayer films which contain ordered layers of more than one protein species were assembled by means of alternate electrostatic adsorption mostly with positively charged poly(ethylenimine) (PEI) or with negatively charged poly(styrenesulfonate) (PSS). Water-soluble proteins used are cytochrome c (Cyt), myoglobin (Mb), lysozyme (Lys), histone f3, hemoglobin (Hb), glucoamylase (GA), and glucose oxidase (GOD). Charged protein layers formed multilayers with linear polymers acting as glue or filler. The assembly was monitored by a quartz crystal microbalance and UV spectroscopy. Linear film growth was observed up to at least 25 molecular layers. The assembly of Mb and Lys, both positively-charged, was realized in alternation with PSS in the form of {PEI/PSS + (Mb/PSS)(2) + (Mb/PSS/Lys/PSS)(4)}. The assembly of oppositely-charged (at pH 6.5) Lys and GOD consists from Lys and GOD layers separated by a polycation/polyanion bilayer: {PEI/PSS/PEI + (PSS/Lys)(2) + PSS/PEI + (GOD/PEI)(6)}. Hb was assembled as ''positive'' unit at pH 4.5 (in alternation with PSS) and as ''negative'' unit at pH 9.2 (in alternation with PEI). A multilayer consisting of alternating montmorillonite, PEI, and GOD layers was also assembled. These biomolecular architecture open a way to construct artificially orchestrated protein systems that can carry out complex enzymic reactions.
Document Type: Article
Addresses: RES DEV CORP JAPAN,SUPERMOLECULES PROJECT,KURUME,FUKUOKA 830,JAPAN.
KYUSHU UNIV,FAC ENGN,FUKUOKA 812,JAPAN.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: The structure of catalytically active gold on titania
Author(s): Chen, MS (Chen, MS); Goodman, DW (Goodman, DW)
Source: SCIENCE Volume: 306 Issue: 5694 Pages: 252-255 DOI: 10.1126/science.1102420 Published: OCT 8 2004
Times Cited in Web of Science Core Collection: 1257
Total Times Cited: 1278
Abstract: The high catalytic activity of gold clusters on oxides has been attributed to structural effects (including particle thickness and shape and metal oxidation state), as well as to support effects. We have created well-ordered gold monolayers and bilayers that completely wet (cover) the oxide support, thus eliminating particle shape and direct support effects. High-resolution electron energy loss spectroscopy and carbon monoxide adsorption confirm that the gold atoms are bonded to titanium atoms. Kinetic measurements for the catalytic oxidation of carbon monoxide show that the gold bilayer structure is significantly more active (by more than an order of magnitude) than the monolayer.
Document Type: Article
Addresses: Texas A&M Univ, Dept Chem, College Stn, TX 77842 USA.
Reprint Address: Goodman, DW (reprint author), Texas A&M Univ, Dept Chem, College Stn, TX 77842 USA.
E-mail Addresses: goodman@mail.chem.tamu.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
eISSN: 1095-9203
Title: Ordered mesoporous carbons
Author(s): Ryoo, R (Ryoo, R); Joo, SH (Joo, SH); Kruk, M (Kruk, M); Jaroniec, M (Jaroniec, M)
Source: ADVANCED MATERIALS Volume: 13 Issue: 9 Pages: 677-681 DOI: 10.1002/1521-4095(200105)13:9<677::AID-ADMA677>3.0.CO;2-C Published: MAY 3 2001
Times Cited in Web of Science Core Collection: 1257
Total Times Cited: 1288
Abstract: Ordered mesoporous carbons have recently been synthesized using ordered mesoporous silica templates. The synthesis procedure involves infiltration of the pores of the template with appropriate carbon precursor, its carbonization, and subsequent template removal. The template needs to exhibit three-dimensional pore structure in order to be suitable for the ordered mesoporous carbon synthesis, otherwise disordered microporous carbon is formed. MCM-48, SBA-1, and SBA-15 silicas were successfully used to synthesize carbons with cubic or hexagonal frameworks, narrow mesopore size distributions, high nitrogen Brunauer-Emmett-Teller (BET) specific surface areas (up to 1800 m(2) g(-1)), and large pore volumes. Ordered mesoporous carbons are promising in many applications, including adsorption of large molecules, chromatography, and manufacturing of electrochemical double-layer capacitors.
Document Type: Article
Addresses: Korea Adv Inst Sci & Technol, Dept Chem, Sch Mol Sci BK21, Taejon 305701, South Korea.
Kent State Univ, Dept Chem, Kent, OH 44242 USA.
Reprint Address: Ryoo, R (reprint author), Korea Adv Inst Sci & Technol, Dept Chem, Sch Mol Sci BK21, Taejon 305701, South Korea.
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Nanoscience & Nanotechnology; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 0935-9648
Title: A survey of structure-property relationships of surfaces that resist the adsorption of protein
Author(s): Ostuni, E (Ostuni, E); Chapman, RG (Chapman, RG); Holmlin, RE (Holmlin, RE); Takayama, S (Takayama, S); Whitesides, GM (Whitesides, GM)
Source: LANGMUIR Volume: 17 Issue: 18 Pages: 5605-5620 DOI: 10.1021/la010384m Published: SEP 4 2001
Times Cited in Web of Science Core Collection: 1234
Total Times Cited: 1258
Abstract: This paper describes the use of surface plasmon resonance (SPR) spectroscopy and self-assembled monolayers (SAMs) to determine the characteristics of functional groups that give surfaces the ability to resist the nonspecific adsorption of proteins from solution. Mixed SAMs presenting different functional groups were prepared for screening using a synthetic protocol based on the reaction of organic amines with a SAM terminated by interchain carboxylic anhydride groups. Surfaces that presented derivatives of oligo(sarcosine), N-acetylpiperazine, and permethylated sorbitol groups were particularly effective in resisting the adsorption of proteins. Incorporation of these groups into single-component SAMs resulted in surfaces that are comparable to (but slightly less good than) single-component SAMs that present oligo(ethylene glycol) in their ability to resist the adsorption of proteins. In the group of surfaces examined, those that resisted the adsorption of proteins had the following properties: they were hydrophilic; they contained groups that were hydrogen-bond acceptors but not hydrogen-bond donors; and they were overall electrically neutral.
Document Type: Article
Addresses: Harvard Univ, Dept Chem & Chem Biol, Cambridge, MA 02138 USA.
Reprint Address: Whitesides, GM (reprint author), Harvard Univ, Dept Chem & Chem Biol, 12 Oxford St, Cambridge, MA 02138 USA.
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0743-7463
Title: Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction
Author(s): Li, JJ (Li, JJ); Wang, YA (Wang, YA); Guo, WZ (Guo, WZ); Keay, JC (Keay, JC); Mishima, TD (Mishima, TD); Johnson, MB (Johnson, MB); Peng, XG (Peng, XG)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 125 Issue: 41 Pages: 12567-12575 DOI: 10.1021/ja0363563 Published: OCT 15 2003
Times Cited in Web of Science Core Collection: 1231
Total Times Cited: 1257
Abstract: Successive ion layer adsorption and reaction (SILAR) originally developed for the deposition of thin films on solid substrates from solution baths is introduced as a technique for the growth of high-quality core/shell nanocrystals of compound semiconductors. The growth of the shell was designed to grow one monolayer at a time by alternating injections of air-stable and inexpensive cationic and anionic precursors into the reaction mixture with core nanocrystals. The principles of SILAR were demonstrated by the CdSe/CdS core/shell model system using its shell-thickness-dependent optical spectra as the probes with CdO and elemental S as the precursors. For this reaction system, a relatively high temperature, about 220-240 degreesC, was found to be essential for SILAR to fully occur. The synthesis can be readily performed on a multigram scale. The size distribution of the core/shell nanocrystals was maintained even after five monolayers of CdS shell (equivalent to about 10 times volume increase for a 3.5 nm CdSe nanocrystal) were grown onto the core nanocrystals. The epitaxial growth of the core/shell structures was verified by optical spectroscopy, TEM, XRD, and XPS. The photoluminescence quantum yield (PL QY) of the as-prepared CdSe/CdS core/shell nanocrystals ranged from 20% to 40%, and the PL full-width at half-maximum (fwhm) was maintained between 23 and 26 nm, even for those nanocrystals for which the UV-vis and PL peaks red-shifted by about 50 nm from that of the core nanocrystals. Several types of brightening phenomena were observed, some of which can further boost the PL QY of the core/shell nanocrystals. The CdSe/CdS core/shell nanocrystals were found to be superior in comparison to the highly luminescent CdSe plain core nanocrystals. The SILAR technique reported here can also be used for the growth of complex colloidal semiconductor nanostructures, such as quantum shells and colloidal quantum wells.
Document Type: Article
Addresses: Univ Arkansas, Dept Chem & Biochem, Fayetteville, AR 72701 USA.
Univ Oklahoma, Joint MRSEC, Norman, OK 73019 USA.
Univ Arkansas, Nanomat & Nanofabricat Labs, Fayetteville, AR 72703 USA.
Univ Oklahoma, Dept Phys & Astron, Norman, OK 73019 USA.
Reprint Address: Peng, XG (reprint author), Univ Arkansas, Dept Chem & Biochem, Fayetteville, AR 72701 USA.
E-mail Addresses: xpeng@comp.uark.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Water-Dispersible Magnetite-Reduced Graphene Oxide Composites for Arsenic Removal
Author(s): Chandra, V (Chandra, Vimlesh); Park, J (Park, Jaesung); Chun, Y (Chun, Young); Lee, JW (Lee, Jung Woo); Hwang, IC (Hwang, In-Chul); Kim, KS (Kim, Kwang S.)
Source: ACS NANO Volume: 4 Issue: 7 Pages: 3979-3986 DOI: 10.1021/nn1008897 Published: JUL 2010
Times Cited in Web of Science Core Collection: 1228
Total Times Cited: 1261
Abstract: Magnetite-graphene hybrids have been synthesized via a chemical reaction with a magnetite particle size of similar to 10 nm. The composites are superparamagnetic at room temperature and can be separated by an external magnetic field. As compared to bare magnetite particles, the hybrids show a high binding capacity for As(III) and As(V), whose presence in the drinking water in wide areas of South Asia has been a huge problem. Their high binding capacity is due to the increased adsorption sites in the M-RGO composite which occurs by reducing the aggregation of bare magnetite. Since the composites show near complete (over 99.9%) arsenic removal within 1 ppb, they are practically usable for arsenic separation from water.
Document Type: Article
Addresses: [Chandra, Vimlesh; Park, Jaesung; Chun, Young; Lee, Jung Woo; Hwang, In-Chul; Kim, Kwang S.] Pohang Univ Sci & Technol, Dept Chem, Ctr Superfunct Mat, Pohang 790784, South Korea.
Reprint Address: Kim, KS (reprint author), Pohang Univ Sci & Technol, Dept Chem, Ctr Superfunct Mat, Pohang 790784, South Korea.
E-mail Addresses: spfe@postech.ac.kr; kim@postech.ac.kr
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Nanoscience & Nanotechnology; Materials Science, Multidisciplinary
ISSN: 1936-0851
eISSN: 1936-086X
Title: Trends in the exchange current for hydrogen evolution
Author(s): Norskov, JK (Norskov, JK); Bligaard, T (Bligaard, T); Logadottir, A (Logadottir, A); Kitchin, JR (Kitchin, JR); Chen, JG (Chen, JG); Pandelov, S (Pandelov, S); Norskov, JK (Norskov, JK)
Source: JOURNAL OF THE ELECTROCHEMICAL SOCIETY Volume: 152 Issue: 3 Pages: J23-J26 DOI: 10.1149/1.1856988 Published: 2005
Times Cited in Web of Science Core Collection: 1214
Total Times Cited: 1222
Abstract: A density functional theory database of hydrogen chemisorption energies on close packed surfaces of a number of transition and noble metals is presented. The bond energies are used to understand the trends in the exchange current for hydrogen evolution. A volcano curve is obtained when measured exchange currents are plotted as a function of the calculated hydrogen adsorption energies and a simple kinetic model is developed to understand the origin of the volcano. The volcano curve is also consistent with Pt being the most efficient electrocatalyst for hydrogen evolution. (c) 2005 The Electrochemical Society. [DOI: 10.1149/1.1856988] All rights reserved.
Document Type: Article
Addresses: Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Phys, DK-2800 Lyngby, Denmark.
Univ Delaware, Dept Chem Engn, Newark, DE 19716 USA.
Tech Univ Munich, Dept Phys, D-85748 Garching, Germany.
Reprint Address: Norskov, JK (reprint author), Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Phys, DK-2800 Lyngby, Denmark.
E-mail Addresses: norskov@fysik.dtu.dk
Web of Science Categories: Electrochemistry; Materials Science, Coatings & Films
ISSN: 0013-4651
Title: Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity
Author(s): McFarland, AD (McFarland, AD); Van Duyne, RP (Van Duyne, RP)
Source: NANO LETTERS Volume: 3 Issue: 8 Pages: 1057-1062 DOI: 10.1021/nl034372s Published: AUG 2003
Times Cited in Web of Science Core Collection: 1213
Total Times Cited: 1225
Abstract: This work utilizes dark-field optical microscopy to demonstrate the localized surface plasmon resonance gamma(max) response of individual Ag nanoparticles to the formation of a monolayer of small-molecule adsorbates. The adsorption of fewer than 60 000 1-hexadecanethiol molecules on single Ag nanoparticles results in a localized surface plasmon resonance shift of 40.7 nm. Additionally, the kinetics of the single nanoparticle response was shown to be comparable to that of other real-time sensor technologies.
Document Type: Article
Addresses: Northwestern Univ, Dept Chem, Evanston, IL 60208 USA.
Reprint Address: Van Duyne, RP (reprint author), Northwestern Univ, Dept Chem, 2145 Sheridan Rd, Evanston, IL 60208 USA.
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Nanoscience & Nanotechnology; Materials Science, Multidisciplinary; Physics, Applied; Physics, Condensed Matter
ISSN: 1530-6984
Title: Electronic factors determining the reactivity of metal surfaces
Author(s): Hammer, B (Hammer, B); Norskov, JK (Norskov, JK)
Source: SURFACE SCIENCE Volume: 343 Issue: 3 Pages: 211-220 DOI: 10.1016/0039-6028(96)80007-0 Published: DEC 10 1995
Times Cited in Web of Science Core Collection: 1209
Total Times Cited: 1220
Abstract: Based on density functional theory calculations of H-2 dissociation on Al(111), Cu(111), Pt(111) and Cu3Pt(111) we present a consistent picture of some key physical properties determining the reactivity of metal and alloy surfaces. The four metal surfaces are chosen to represent metals with no d-bands, with filled d-bands and with d-states at the Fermi level. We show that electronic states in the entire valence band of the metal surface are responsible for the reactivity, which consequently cannot be understood solely in terms of the density of states at the Fermi level nor in terms of the empty d-states above it. Rather we suggest that trends in reactivities can be understood in terms of the hybridization energy between the bonding and anti-bonding adsorbate states and the metal d-bands (when present), and we demonstrate that a simple frozen potential based estimate of the hybridization energy correlates well with the calculated variation of the barrier height for the different metal surfaces.
Document Type: Article
Addresses: TECH UNIV DENMARK, DEPT PHYS, DK-2800 LYNGBY, DENMARK.
JOINT RES CTR ATOM TECHNOL, TSUKUBA, IBARAKI 305, JAPAN.
Reprint Address: Hammer, B (reprint author), TECH UNIV DENMARK, CTR ATOM SCALE MAT PHYS, DK-2800 LYNGBY, DENMARK.
Web of Science Categories: Chemistry, Physical; Physics, Condensed Matter
ISSN: 0039-6028
Title: Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts
Author(s): Guo, DH (Guo, Donghui); Shibuya, R (Shibuya, Riku); Akiba, C (Akiba, Chisato); Saji, S (Saji, Shunsuke); Kondo, T (Kondo, Takahiro); Nakamura, J (Nakamura, Junji)
Source: SCIENCE Volume: 351 Issue: 6271 Pages: 361-365 DOI: 10.1126/science.aad0832 Published: JAN 22 2016
Times Cited in Web of Science Core Collection: 1167
Total Times Cited: 1182
Abstract: Nitrogen (N)-doped carbon materials exhibit high electrocatalytic activity for the oxygen reduction reaction (ORR), which is essential for several renewable energy systems. However, the ORR active site (or sites) is unclear, which retards further developments of high-performance catalysts. Here, we characterized the ORR active site by using newly designed graphite (highly oriented pyrolitic graphite) model catalysts with well-defined p conjugation and well-controlled doping of N species. The ORR active site is created by pyridinic N. Carbon dioxide adsorption experiments indicated that pyridinic N also creates Lewis basic sites. The specific activities per pyridinic N in the HOPG model catalysts are comparable with those of N-doped graphene powder catalysts. Thus, the ORR active sites in N-doped carbon materials are carbon atoms with Lewis basicity next to pyridinic N.
Document Type: Article
Addresses: [Guo, Donghui; Kondo, Takahiro; Nakamura, Junji] Univ Tsukuba, Fac Pure & Appl Sci, Tsukuba, Ibaraki 3058573, Japan.
[Shibuya, Riku; Akiba, Chisato; Saji, Shunsuke] Univ Tsukuba, Grad Sch Pure & Appl Sci, Tsukuba, Ibaraki 3058573, Japan.
Reprint Address: Kondo, T (reprint author), Univ Tsukuba, Fac Pure & Appl Sci, 1-1-1 Tennodai, Tsukuba, Ibaraki 3058573, Japan.
E-mail Addresses: takahiro@irns.tsukuba.ac.jp; nakamura@irns.tsukuba.ac.jp
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
eISSN: 1095-9203
Title: ENGINEERING CELL-SHAPE AND FUNCTION
Author(s): SINGHVI, R (SINGHVI, R); KUMAR, A (KUMAR, A); LOPEZ, GP (LOPEZ, GP); STEPHANOPOULOS, GN (STEPHANOPOULOS, GN); WANG, DIC (WANG, DIC); WHITESIDES, GM (WHITESIDES, GM); INGBER, DE (INGBER, DE)
Source: SCIENCE Volume: 264 Issue: 5159 Pages: 696-698 DOI: 10.1126/science.8171320 Published: APR 29 1994
Times Cited in Web of Science Core Collection: 1160
Total Times Cited: 1183
Abstract: An elastomeric stamp, containing defined features on the micrometer scale, was used to imprint gold surfaces with specific patterns of self-assembled monolayers of alkanethiols and, thereby, to create islands of defined shape and size that support extracellular matrix protein adsorption and cell attachment. Through this technique, it was possible to place cells in predetermined locations and arrays, separated by defined distances, and to dictate their shape. Limiting the degree of cell extension provided control over cell growth and protein secretion. This method is experimentally simple and highly adaptable. It should be useful for applications in biotechnology that require analysis of individual cells cultured at high density or repeated access to cells placed in specified locations.
Document Type: Article
Addresses: HARVARD UNIV,DEPT CHEM,CAMBRIDGE,MA 02138.
MIT,CTR BIOTECHNOL PROC ENGN,CAMBRIDGE,MA 02139.
MIT,DEPT CHEM ENGN,CAMBRIDGE,MA 02139.
CHILDRENS HOSP,DEPT SURG,BOSTON,MA 02115.
CHILDRENS HOSP,DEPT PATHOL,BOSTON,MA 02115.
HARVARD UNIV,SCH MED,BOSTON,MA 02115.
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
Title: Kinetics and mechanism of removal of methylene blue by adsorption on various carbons - a comparative study
Author(s): Kannan, N (Kannan, N); Sundaram, MM (Sundaram, MM)
Source: DYES AND PIGMENTS Volume: 51 Issue: 1 Pages: 25-40 DOI: 10.1016/S0143-7208(01)00056-0 Published: OCT 2001
Times Cited in Web of Science Core Collection: 1159
Total Times Cited: 1206
Abstract: The kinetics and mechanism of methylene blue adsorption on commercial activated carbon (CAC) and indigenously prepared activated carbons from bamboo dust, coconut shell, groundnut shell, rice husk, and straw, have been studied. The effects of various experimental parameters have been investigated using a batch adsorption technique to obtain information on treating effluents from the dye industry. The extent of dye removal increased with decrease in the initial concentration of the dye and particle size of the adsorbent and also increased with increase in contact time, amount of adsorbent used and the initial pH of the solution. Adsorption data were modeled using the Freundlich and Langmuir adsorption isotherms and first order kinetic equations. The kinetics of adsorption were found to be first order with regard to intra-particle diffusion rate. The adsorption capacities of indigenous activated carbons have been compared with that of the commercial activated carbon. The results indicate that such carbons could be employed as low cost alternatives to commercial activated carbon in wastewater treatment for the removal of colour and dyes. (C) 2001 Published by Elsevier Science Ltd. All rights reserved.
Document Type: Article
Addresses: Ayya Nadar Janaki Ammal Coll Autonomous, Dept Chem, Sivakasi 626124, Tamil Nadu, India.
Reprint Address: Kannan, N (reprint author), Ayya Nadar Janaki Ammal Coll Autonomous, Dept Chem, Sivakasi 626124, Tamil Nadu, India.
Web of Science Categories: Chemistry, Applied; Engineering, Chemical; Materials Science, Textiles
ISSN: 0143-7208
Title: A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents
Author(s): Ho, YS (Ho, YS); McKay, G (McKay, G)
Source: PROCESS SAFETY AND ENVIRONMENTAL PROTECTION Volume: 76 Issue: B4 Pages: 332-340 DOI: 10.1205/095758298529696 Published: NOV 1998
Times Cited in Web of Science Core Collection: 1155
Total Times Cited: 1189
Abstract: A comparison of kinetic models describing the sorption of pollutants has been reviewed. The rate models evaluated include the Elovich equation, the pseudo-first order equation and the pseudo-second order equation. Results show that chemisorption processes could be rate limiting in the sorption step. The pseudo-second order equation may be applied for chemisorption processes with a high degree of correlation in several Literature cases where a pseudo-first order rate mechanism has been arbitrarily assumed.
Document Type: Article
Addresses: Hong Kong Univ Sci & Technol, Dept Chem Engn, Kowloon, Peoples R China.
Reprint Address: McKay, G (reprint author), Hong Kong Univ Sci & Technol, Dept Chem Engn, Kowloon, Peoples R China.
Web of Science Categories: Engineering, Environmental; Engineering, Chemical
ISSN: 0957-5820
Title: Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements
Author(s): Kruk, M (Kruk, M); Jaroniec, M (Jaroniec, M); Sayari, A (Sayari, A)
Source: LANGMUIR Volume: 13 Issue: 23 Pages: 6267-6273 DOI: 10.1021/la970776m Published: NOV 12 1997
Times Cited in Web of Science Core Collection: 1155
Total Times Cited: 1165
Abstract: MCM-41 siliceous molecular sieves were used to test the applicability of the Kelvin equation for nitrogen adsorption in cylindrical pores of the size from 2 to 6.5 nm. It was shown that the Kelvin equation for the hemispherical meniscus, corrected for the statistical film thickness, is in quite good agreement with an experimental relation between the pore size and the capillary condensation pressure. The agreement can be made quantitative in the pore size range from ca. 2 to 6.5 nm, if a simple correction to the Kelvin equation is introduced. The required statistical film thickness curve (t-curve) was calculated using nitrogen adsorption data for large pore MCM-41 samples and the obtained results were extrapolated using an adsorption isotherm for a macroporous silica gel. Moreover, an accurate analytical representation of the t-curve was found. Since both the corrected Kelvin equation for cylindrical pores and the t-curve have simple analytical forms, they can conveniently be used in a variety of methods to evaluate porosity. It was shown that the BJH method with the corrected Kelvin equation accurately reproduces pore sizes of MCM-41 materials. A comparison was made between the specific surface areas for the MCM-41 samples calculated on the basis of the BET equation and those obtained using other independent methods. The results strongly suggest that when nitrogen adsorption data are used, the BET method overestimates the specific surface area of siliceous materials. The latter conclusion was supported by the examination of the obtained statistical film thickness curve.
Document Type: Article
Addresses: KENT STATE UNIV, DEPT CHEM, KENT, OH 44242 USA.
UNIV LAVAL, DEPT CHEM ENGN, ST FOY, PQ G1K 7P4, CANADA.
UNIV LAVAL, CERPIC, ST FOY, PQ G1K 7P4, CANADA.
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0743-7463
Title: Advances in the catalysis of Au nanoparticles
Author(s): Haruta, M (Haruta, M); Date, M (Date, M)
Source: APPLIED CATALYSIS A-GENERAL Volume: 222 Issue: 1-2 Pages: 427-437 DOI: 10.1016/S0926-860X(01)00847-X Published: DEC 20 2001
Times Cited in Web of Science Core Collection: 1146
Total Times Cited: 1184
Abstract: Gold catalysts have recently been attracting rapidly growing interests due to their potential applicabilities to many reactions of both industrial and environmental importance. This article reviews the latest advances in the catalysis research on Au. For low-temperature CO oxidation mechanistic arguments are summarized, focusing on Au/TiO2 together with the effect of preparation conditions and pretreatments. The quantum size effect is also discussed in the adsorption and reaction of CO over Au clusters smaller than 2 nm. in diameter. In addition, recent developments are introduced in the epoxidation of propylene, water-gas-shift reaction, hydrogenation of unsaturated hydrocarbons, and liquid-phase selective oxidation. The role of perimeter interface between Au particles and the support is emphasized as a unique reaction site for the reactants adsorbed separately, one on Au and another on the support surfaces. (C) 2001 Elsevier Science B.V. All rights reserved.
Document Type: Article
Addresses: Inst Adv Ind Sci & Technol, Res Inst Green Technol, Tsukuba, Ibaraki 3058569, Japan.
Ind Adv Ind Sci & Technol, Special Div Green Life Technol, Ikeda, Osaka 5638577, Japan.
Reprint Address: Haruta, M (reprint author), Inst Adv Ind Sci & Technol, Res Inst Green Technol, 16-1 Onogawa, Tsukuba, Ibaraki 3058569, Japan.
Web of Science Categories: Chemistry, Physical; Environmental Sciences
ISSN: 0926-860X
Title: Doping semiconductor nanocrystals
Author(s): Erwin, SC (Erwin, SC); Zu, LJ (Zu, LJ); Haftel, MI (Haftel, MI); Efros, AL (Efros, AL); Kennedy, TA (Kennedy, TA); Norris, DJ (Norris, DJ)
Source: NATURE Volume: 436 Issue: 7047 Pages: 91-94 DOI: 10.1038/nature03832 Published: JUL 7 2005
Times Cited in Web of Science Core Collection: 1143
Total Times Cited: 1158
Abstract: Doping-the intentional introduction of impurities into a material-is fundamental to controlling the properties of bulk semiconductors. This has stimulated similar efforts to dope semiconductor nanocrystals(1-4). Despite some successes(5-11), many of these efforts have failed, for reasons that remain unclear. For example, Mn can be incorporated into nanocrystals of CdS and ZnSe (refs 7-9), but not into CdSe (ref. 12)-despite comparable bulk solubilities of near 50 per cent. These difficulties, which have hindered development of new nanocrystalline materials(13-15), are often attributed to 'self-purification', an allegedly intrinsic mechanism whereby impurities are expelled. Here we show instead that the underlying mechanism that controls doping is the initial adsorption of impurities on the nanocrystal surface during growth. We find that adsorption-and therefore doping efficiency-is determined by three main factors: surface morphology, nanocrystal shape, and surfactants in the growth solution. Calculated Mn adsorption energies and equilibrium shapes for several nanocrystals lead to specific doping predictions. These are confirmed by measuring how the Mn concentration in ZnSe varies with nanocrystal size and shape. Finally, we use our predictions to incorporate Mn into previously undopable CdSe nanocrystals. This success establishes that earlier difficulties with doping are not intrinsic, and suggests that a variety of doped nanocrystals-for applications from solar cells(16) to spintronics(17)-can be anticipated.
Document Type: Article
Addresses: USN, Res Lab, Washington, DC 20375 USA.
Univ Minnesota, Dept Chem Engn & Mat Sci, Minneapolis, MN 55455 USA.
Reprint Address: Erwin, SC (reprint author), USN, Res Lab, Washington, DC 20375 USA.
E-mail Addresses: Steven.Erwin@nrl.navy.mil; dnorris@umn.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
eISSN: 1476-4687
Title: Characterization of DNA probes immobilized on gold surfaces
Author(s): Herne, TM (Herne, TM); Tarlov, MJ (Tarlov, MJ)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 119 Issue: 38 Pages: 8916-8920 DOI: 10.1021/ja9719586 Published: SEP 24 1997
Times Cited in Web of Science Core Collection: 1142
Total Times Cited: 1164
Abstract: We have characterized thiol-derivatized, single-stranded DNA (5'-HS-(CH2)(6)-CAC GAC GTT GTA AAA CGA CGG CCA G-3', abbreviated HS-ssDNA) attached to gold via a sulfur-gold linkage using X-ray photoelectron spectroscopy (XPS), ellipsometry, and P-32-radiolabeling experiments. We found that hybridization of surface-bound HS-ssDNA is dependent on surface coverage. The buffer concentration of the HS-ssDNA solution was found to have a profound effect on surface coverage, with adsorption greatly reduced at low salt concentration. More precise control over surface coverage was achieved by creating mixed monolayers of the thiol-derivatized probe and a spacer thiol, mercaptohexanol (MCH), by way of a two-step method, where first the gold substrate is exposed to a micromolar solution of HS-ssDNA, followed by exposure to a millimolar solution of MCH. A primary advantage of using this two-step process to form HS-ssDNA/MCH mixed monolayers is that nonspecifically adsorbed DNA is largely removed from the surface. Thus, the majority of surface-bound probes are accessible for specific hybridization with complementary oligonucleotides and are able to discriminate between complementary and noncomplementary target molecules. Moreover, the probe-modified surfaces were found to be stable, and hybridization reactions were found to be completely reversible and specific in a series of experiments where duplex melting was examined.
Document Type: Article
Reprint Address: Herne, TM (reprint author), NATL INST STAND & TECHNOL,CHEM SCI & TECHNOL LAB,GAITHERSBURG,MD 20899, USA.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Preparation and Enhanced Visible-Light Photocatalytic H-2-Production Activity of Graphene/C3N4 Composites
Author(s): Xiang, QJ (Xiang, Quanjun); Yu, JG (Yu, Jiaguo); Jaroniec, M (Jaroniec, Mietek)
Source: JOURNAL OF PHYSICAL CHEMISTRY C Volume: 115 Issue: 15 Pages: 7355-7363 DOI: 10.1021/jp200953k Published: APR 21 2011
Times Cited in Web of Science Core Collection: 1137
Total Times Cited: 1161
Abstract: Graphene and graphitic carbon nitride (g-C3N4) composite photocatalysts were prepared by a combined impregnation-chemical reduction strategy involving polymerization of melamine in the presence of graphene oxide (precursors) and hydrazine hydrate (reducing agent), followed by thermal treatment at 550 degrees C under flowing nitrogen. The resulting, graphene/g-C3N4 composite photocatalysts were characterized by X-ray diffraction, transmission electron microscopy, UV-visible spectrophotometry, nitrogen adsorption, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, Raman spectroscopy, and photoluminescence spectroscopy. The transient photocurrent response was measured for several on-off cycles of intermittent irradiation. The effect of graphene content on the rate of visible-light photocatalytic hydrogen production was studied for a series of graphene-graphitic carbon nitride composite samples containing Pt as a cocatalyst in methanol aqueous solutions. This study shows that graphene sheets act as electronic conductive channels to efficiently separate the photogenerated charge carriers and, consequently, to enhance the visible-light photocatalytic H-2-production activity of g-C3N4. The optimal graphene content was determined to be similar to 1.0 wt %, and the corresponding H-2-production rate was 451 mu mol h(-1) g(-1), which exceeded that of pure g-C3N4 by more than 3.07 times. The proposed mechanism for the enhanced visible-light photocatalytic activity of g-C3N4 modified by a 5 mall amount of graphene was further confirmed by photoluminescence spectroscopy and transient photocurrent response. The metal-free graphene/g-C3N4 composites showed high visible-light photocatalytic activity, which makes them promising nanomaterials for further applications in water treatment and dye-sensitized solar cells.
Document Type: Article
Addresses: [Xiang, Quanjun; Yu, Jiaguo] Wuhan Univ Technol, State Key Lab Adv Technol Mat Synth & Proc, Luoshi Rd 122, Wuhan 430070, Peoples R China.
[Jaroniec, Mietek] Kent State Univ, Dept Chem, Kent, OH 44242 USA.
Reprint Address: Yu, JG (reprint author), Wuhan Univ Technol, State Key Lab Adv Technol Mat Synth & Proc, Luoshi Rd 122, Wuhan 430070, Peoples R China.
E-mail Addresses: jiaguoyu@yahoo.com; jaroniec@kent.edu
Web of Science Categories: Chemistry, Physical; Nanoscience & Nanotechnology; Materials Science, Multidisciplinary
ISSN: 1932-7447
Title: Removal of nutrients in various types of constructed wetlands
Author(s): Vymazal, J (Vymazal, Jan)
Source: SCIENCE OF THE TOTAL ENVIRONMENT Volume: 380 Issue: 1-3 Special Issue: SI Pages: 48-65 DOI: 10.1016/j.scitotenv.2006.09.014 Published: JUL 15 2007
Times Cited in Web of Science Core Collection: 1135
Total Times Cited: 1318
Abstract: The processes that affect removal and retention of nitrogen during wastewater treatment in constructed wetlands (CWs) are manifold and include NH(3) volatilization, nitrification, denitrification, nitrogen fixation, plant and microbial uptake, mineralization (ammonification), nitrate reduction to ammonium (nitrate-ammonification), anaerobic ammonia oxidation (ANAMMOX), fragmentation, sorption, desorption, burial, and leaching. However, only few processes ultimately remove total nitrogen from the wastewater while most processes just convert nitrogen to its various forms. Removal of total nitrogen in studied types of constructed wetlands varied between 40 and 55% with removed load ranging between 250 and 630 g N m(-2) yr(-1) depending on CWs type and inflow loading. However, the processes responsible for the removal differ in magnitude among systems. Single-stage constructed wetlands cannot achieve high removal of total nitrogen due to their inability to provide both aerobic and anaerobic conditions at the same time. Vertical flow constructed wetlands remove successfully ammonia-N but very limited denitrification takes place in these systems. On the other hand, horizontal-flow constructed wetlands provide good conditions for dentrification but the ability of these system to nitrify ammonia is very limited. Therefore, various types of constructed wetlands may be combined with each other in order to exploit the specific advantages of the individual systems. The soil phosphorus cycle is fundamentally different from the N cycle. There are no valency changes during biotic assimilation of inorganic P or during decomposition of organic P by microorganisms. Phosphorus transformations during wastewater treatment in CWs include adsorption, desorption, precipitation, dissolution, plant and microbial uptake, fragmentation, leaching, mineralization, sedimentation (peat accretion) and burial. The major phosphorus removal processes are sorption, precipitation, plant uptake (with subsequent harvest) and peat/soil accretion. However, the first three processes are saturable and soil accretion occurs only in FWS CWs. Removal of phosphorus in all types of constructed wetlands is low unless special substrates with high sorption capacity are used. Removal of total phosphorus varied between 40 and 60% in all types of constructed wetlands with removed load ranging between 45 and 75 g N m-2 yr 1 depending on CWs type and inflow loading. Removal of both nitrogen and phosphorus via harvesting of abroveground biomass of emergent vegetation is low but it could be substantial for lightly loaded systems (cca 100-200 g N m(-2) yr(-1) and 10-20 g P m(-2) yr(-1)). Systems with free-floating plants may achieve higher removal of nitrogen via harvesting due to multiple harvesting schedule. (C) 2006 Elsevier BN. All rights reserved.
Document Type: Article
Addresses: ENKI Ops, Prague 16900 6, Czech Republic.
Duke Univ, Wetland Ctr, Nicholas Sch Environm & Earth Sci, Durham, NC 27708 USA.
Reprint Address: Vymazal, J (reprint author), ENKI Ops, Ricanova 40, Prague 16900 6, Czech Republic.
E-mail Addresses: vymazal@yahoo.com
Web of Science Categories: Environmental Sciences
ISSN: 0048-9697
Title: Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation
Author(s): Nugent, P (Nugent, Patrick); Belmabkhout, Y (Belmabkhout, Youssef); Burd, SD (Burd, Stephen D.); Cairns, AJ (Cairns, Amy J.); Luebke, R (Luebke, Ryan); Forrest, K (Forrest, Katherine); Pham, T (Pham, Tony); Ma, SQ (Ma, Shengqian); Space, B (Space, Brian); Wojtas, L (Wojtas, Lukasz); Eddaoudi, M (Eddaoudi, Mohamed); Zaworotko, MJ (Zaworotko, Michael J.)
Source: NATURE Volume: 495 Issue: 7439 Pages: 80-84 DOI: 10.1038/nature11893 Published: MAR 7 2013
Times Cited in Web of Science Core Collection: 1128
Total Times Cited: 1142
Abstract: The energy costs associated with the separation and purification of industrial commodities, such as gases, fine chemicals and fresh water, currently represent around 15 per cent of global energy production, and the demand for such commodities is projected to triple by 2050 (ref. 1). The challenge of developing effective separation and purification technologies that have much smaller energy footprints is greater for carbon dioxide (CO2) than for other gases; in addition to its involvement in climate change, CO2, is an impurity in natural gas, biogas (natural gas produced from biomass), syngas (CO/H-2, the main source of hydrogen in refineries) and many other gas streams. In the context of porous crystalline materials that can exploit both equilibrium and kinetic selectivity, size selectivity and targeted molecular recognition are attractive characteristics for CO2 separation and capture, as exemplified by zeolites 5A and 13X (ref. 2), as well as metal-organic materials (MOMs)(3-9). Here we report that a crystal engineering(7) or reticular chemistry(5,9) strategy that controls pore functionality and size in a series of MOMs with coordinately saturated metal centres and periodically arrayed hexafluorosilicate (SiF62-) anions enables a 'sweet spot' of kinetics and thermodynamics that offers high volumetric uptake at low CO2 partial pressure (less than 0.15 bar). Most importantly, such MOMs offer an unprecedented CO2 sorption selectivity over N-2, H-2 and CH4, even in the presence of moisture. These MOMs are therefore relevant to CO2 separation in the context of post-combustion (flue gas, CO2/N-2), pre-combustion (shifted synthesis gas stream, CO2/H-2) and natural gas upgrading (natural gas clean-up, CO2/CH4).
Document Type: Article
Addresses: [Nugent, Patrick; Burd, Stephen D.; Forrest, Katherine; Pham, Tony; Ma, Shengqian; Space, Brian; Wojtas, Lukasz; Eddaoudi, Mohamed; Zaworotko, Michael J.] Univ S Florida, Dept Chem, Tampa, FL 33620 USA.
[Belmabkhout, Youssef; Cairns, Amy J.; Luebke, Ryan; Eddaoudi, Mohamed] 4700 King Abdullah Univ Sci & Technol KAUST, Div Phys Sci & Engn, Adv Membranes & Porous Mat Ctr, Thuwal 239556900, Saudi Arabia.
Reprint Address: Eddaoudi, M (reprint author), Univ S Florida, Dept Chem, 4202 East Fowler Ave, Tampa, FL 33620 USA.
E-mail Addresses: mohamed.eddaoudi@kaust.edu.sa; xtal@usf.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
eISSN: 1476-4687
Title: GENERAL TREATMENT AND CLASSIFICATION OF SOLUTE ADSORPTION-ISOTHERM .1. THEORETICAL
Author(s): GILES, CH (GILES, CH); SMITH, D (SMITH, D); HUITSON, A (HUITSON, A)
Source: JOURNAL OF COLLOID AND INTERFACE SCIENCE Volume: 47 Issue: 3 Pages: 755-765 DOI: 10.1016/0021-9797(74)90252-5 Published: 1974
Times Cited in Web of Science Core Collection: 1126
Total Times Cited: 1162
Document Type: Article
Addresses: UNIV STRATHCLYDE,DEPT PURE & APPL CHEM,GLASGOW G1,SCOTLAND.
UNIV STRATHCLYDE,DEPT MATH,GLASGOW G1,SCOTLAND.
Web of Science Categories: Chemistry, Physical
ISSN: 0021-9797
Title: Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or Cr-III(OH)center dot{O2C-C6H4-CO2}center dot{HO2C-C6H4-CO2H}(x)center dot H2Oy
Author(s): Serre, C (Serre, C); Millange, F (Millange, F); Thouvenot, C (Thouvenot, C); Nogues, M (Nogues, M); Marsolier, G (Marsolier, G); Louer, D (Louer, D); Ferey, G (Ferey, G)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 124 Issue: 45 Pages: 13519-13526 DOI: 10.1021/ja0276974 Published: NOV 13 2002
Times Cited in Web of Science Core Collection: 1123
Total Times Cited: 1136
Abstract: The first three-dimensional chromium(III) dicarboxylate, MIL-53as or Cr-III(OH) (.) {O2C-C6H4-CO2}. has been obtained under hydrothermal conditions (as: as-synthesized). The free acid can be removed by calcination giving the resulting solid, MIL-53ht or Cr-III(OH).{O2C-C6H4-CO2}. At room temperature, MIL-53ht adsorbs atmospheric water immediately to give Cr-III(OH).[O2C - C6H4-CO2}.H2O or MIL-53lt (It: low-temperature form, ht: high-temperature form). Both structures, which have been determined by using X-ray powder diffraction data, are built up from chains of chromium(Ill) octahedra linked through terephthalate dianions. This creates a three-dimensional structure with an array of one-dimensional large pore channels filled with free disordered terephthalic molecules (MIL-53as) or water molecules (MIL-53lt); when the free molecules are removed, this leads to a nanoporous solid (MIL-53ht) with a Langmuir surface area over 1500 m(2)/g. The transition between the hydrated form (MIL-53lt) and the anhydrous solid (MIL-53ht) is fully reversible and followed by a very high breathing effect (more than 5 A), the pores being clipped in the presence of water molecules (MIL-53It) and reopened when the channels are empty (MIL-53ht). The thermal behavior of the two solids has been investigated using TGA and X-ray thermodiffractometry. The sorption properties of MIL-53It have also been studied using several organic solvents. Finally, magnetism measurements performed on MIL-53as and MIL-53It revealed that these two phases are antiferromagnetic with Neel temperatures T-N of 65 and 55 K, respectively. Crystal data for MIL-53as is as follows: orthorhombic space group P-nam with a = 17.340(1) A, b = 12.178(1) Angstrom, c = 6.822(1) Angstrom, and Z = 4. Crystal data for MIL-53ht is as follows: orthorhombic space group Imcm with a = 16.733(1) Angstrom, b = 13.038(1) Angstrom, c = 6.812(1) Angstrom, and Z= 4. Crystal data for MIL-53It is as follows: monoclinic space group C-2/c with a = 19.685(4) Angstrom, b = 7.849(1) Angstrom, beta = 6.782(1) Angstrom, beta = 104.90(1)degrees, and Z = 4.
Document Type: Article
Addresses: Univ Versailles, Inst Lavoisier Franklin, UFR 2483, F-78035 Versailles, France.
Univ Rennes 1, Chim Solide & Inorgan Mol Lab, CNRS, UMR 6511, F-35042 Rennes, France.
Reprint Address: Serre, C (reprint author), Univ Versailles, Inst Lavoisier Franklin, UFR 2483, 45 Ave Etats Unis, F-78035 Versailles, France.
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: PATTERNING SELF-ASSEMBLED MONOLAYERS - APPLICATIONS IN MATERIALS SCIENCE
Author(s): KUMAR, A (KUMAR, A); BIEBUYCK, HA (BIEBUYCK, HA); WHITESIDES, GM (WHITESIDES, GM)
Source: LANGMUIR Volume: 10 Issue: 5 Pages: 1498-1511 DOI: 10.1021/la00017a030 Published: MAY 1994
Times Cited in Web of Science Core Collection: 1106
Total Times Cited: 1115
Abstract: This paper describes an experimentally simple technique, based on stamping or contact printing, to pattern the adsorption of alkanethiolates on surfaces of gold, on scales from 0.2 to 100 mum, and illustrates the use of these patterned surfaces. An elastomeric stamp, fabricated from poly(dimethylsiloxane)(PDMS), was used to deliver alkanethiol to predetermined regions of the surface of an evaporated gold film. With this technique, organic surfaces patterned with well-defined regions exhibiting different chemical and physical properties have been produced. This technique was used to pattern the adsorption of single and multiple SAMs on a single substrate. This method of preparing patterned surfaces has been used in a variety of applications, including the preparation of well-defined, heterogeneous substrates for scanning probe microscopies, the formation of microelectrodes, the formation of microstructures of silicon, the preparation of substrates for the study of condensation figures, and the preparation of substrates for patterned formation of microcrystals.
Document Type: Article
Addresses: HARVARD UNIV,DEPT CHEM,12 OXFORD ST,CAMBRIDGE,MA 02138.
Web of Science Categories: Chemistry, Multidisciplinary; Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0743-7463
Title: PHYSICAL ADSORPTION ON NON-UNIFORM SURFACES
Author(s): HALSEY, G (HALSEY, G)
Source: JOURNAL OF CHEMICAL PHYSICS Volume: 16 Issue: 10 Pages: 931-937 DOI: 10.1063/1.1746689 Published: 1948
Times Cited in Web of Science Core Collection: 1102
Total Times Cited: 1122
Document Type: Article
Web of Science Categories: Chemistry, Physical; Physics, Atomic, Molecular & Chemical
ISSN: 0021-9606
Title: Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces
Author(s): Man, IC (Man, Isabela C.); Su, HY (Su, Hai-Yan); Calle-Vallejo, F (Calle-Vallejo, Federico); Hansen, HA (Hansen, Heine A.); Martinez, JI (Martinez, Jose I.); Inoglu, NG (Inoglu, Nilay G.); Kitchin, J (Kitchin, John); Jaramillo, TF (Jaramillo, Thomas F.); Norskov, JK (Norskov, Jens K.); Rossmeisl, J (Rossmeisl, Jan)
Source: CHEMCATCHEM Volume: 3 Issue: 7 Pages: 1159-1165 DOI: 10.1002/cctc.201000397 Published: JUL 2011
Times Cited in Web of Science Core Collection: 1091
Total Times Cited: 1094
Abstract: Trends in electrocatalytic activity of the oxygen evolution reaction (OER) are investigated on the basis of a large database of HO* and HOO* adsorption energies on oxide surfaces. The theoretical overpotential was calculated by applying standard density functional theory in combination with the computational standard hydrogen electrode (SHE) model. We showed that by the discovery of a universal scaling relation between the adsorption energies of HOO* vs HO*, it is possible to analyze the reaction free energy diagrams of all the oxides in a general way. This gave rise to an activity volcano that was the same for a wide variety of oxide catalyst materials and a universal descriptor for the oxygen evolution activity, which suggests a fundamental limitation on the maximum oxygen evolution activity of planar oxide catalysts.
Document Type: Article
Addresses: [Man, Isabela C.; Su, Hai-Yan; Calle-Vallejo, Federico; Rossmeisl, Jan] Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Design, DK-2800 Lyngby, Denmark.
[Hansen, Heine A.] Northwestern Univ, Dept Mat Sci & Engn, Evanston, IL 60208 USA.
[Martinez, Jose I.] Univ Autonoma Madrid, Dpto Fis Teor Mat Condensada, E-28049 Madrid, Spain.
[Inoglu, Nilay G.; Kitchin, John] Carnegie Mellon Univ, Dept Chem Engn, Pittsburgh, PA 15213 USA.
[Jaramillo, Thomas F.; Norskov, Jens K.] Stanford Univ, Dept Chem Engn, Stanford, CA 94305 USA.
[Norskov, Jens K.] SLAC Natl Accelerator Lab, Stanford, CA 94025 USA.
Reprint Address: Rossmeisl, J (reprint author), Tech Univ Denmark, Dept Phys, Ctr Atom Scale Mat Design, DK-2800 Lyngby, Denmark.
E-mail Addresses: jross@fysik.dtu.dk
Web of Science Categories: Chemistry, Physical
ISSN: 1867-3880
eISSN: 1867-3899
Title: Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-benzenedicarboxylate)(3) (MOF-5)
Author(s): Kaye, SS (Kaye, Steven S.); Dailly, A (Dailly, Anne); Yaghi, OM (Yaghi, Omar M.); Long, JR (Long, Jeffrey R.)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 129 Issue: 46 Pages: 14176-+ DOI: 10.1021/ja076877g Published: NOV 21 2007
Times Cited in Web of Science Core Collection: 1084
Total Times Cited: 1103
Abstract: The prototypical metal-organic framework Zn4O(BDC)(3) (MOF-5, BDC2- = 1,4-benzenedicarboxylate) decomposes gradually in humid air to form a nonporous solid. Recognizing this, improved procedures for its synthesis and handling were developed, leading to significant increases in N-2 and H-2 gas adsorption capacities. Nitrogen adsorption isotherms measured at 77 K reveal an enhanced maximum N-2 uptake of 44.5 mmol/g and a BET surface area of 3800 m(2)/g, compared to the 35.8 mmol/g and 3100 m(2)/g obtained for a sample prepared using previous methods. High-pressure H-2 adsorption isotherms show improvements from 5.0 to 7.1 excess wt % at 77 K and 40 bar. The total H-2 uptake was further observed to climb to 11.5 wt % at 170 bar, corresponding to a volumetric storage density of 77 g/L. Thus, the air-free compound exhibits the highest gravimetric and volumetric H-2 uptake capacities yet demonstrated for a cryogenic hydrogen storage material. Moreover, no loss of capacity was apparent during 24 complete adsorption-desorption cycles, while kinetics measurements showed a loading time of 2 min with application of just 45 bar of pressure.
Document Type: Article
Addresses: Univ Calif Berkeley, Dept Chem, Berkeley, CA 94720 USA.
GM Corp, Chem & Environm Sci Lab, Warren, MI 48090 USA.
Purdue Univ, Coll Engn, W Lafayette, IN 47907 USA.
Univ Calif Los Angeles, Dept Chem & Biochem, Calif NanoSyst Inst, Los Angeles, CA 90095 USA.
Univ Calif Los Angeles, Ctr Reticular Chem, Calif NanoSyst Inst, Los Angeles, CA 90095 USA.
Reprint Address: Long, JR (reprint author), Univ Calif Berkeley, Dept Chem, Berkeley, CA 94720 USA.
E-mail Addresses: jrlong@berkeley.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Nanoparticle silver released into water from commercially available sock fabrics
Author(s): Benn, TM (Benn, Troy M.); Westerhoff, P (Westerhoff, Paul)
Source: ENVIRONMENTAL SCIENCE & TECHNOLOGY Volume: 42 Issue: 11 Pages: 4133-4139 DOI: 10.1021/es7032718 Published: JUN 1 2008
Times Cited in Web of Science Core Collection: 1077
Total Times Cited: 1110
Abstract: Manufacturers of clothing articles employ nanosilver (n-Ag) as an antimicrobial agent, but the environmental impacts of n-Ag release from commercial products are unknown. The quantity and form of the nanomaterials released from consumer products should be determined to assess the environmental risks of nanotechnology. This paper investigates silver released from commercial clothing (socks) into water, and its fate in wastewater treatment plants (WWTPs). Six types of socks contained up to a maximum of 1360 mu g-Ag/g-sock and leached as much as 650 mu g of silver in 500 mL of distilled water. Microscopy conducted on sock material and wash water revealed the presence of silver particles from 10 to 500 nm in diameter. Physical separation and ion selective electrode (ISE) analyses suggest that both colloidal and ionic silver leach from the socks. Variable leaching rates among sock types suggests that the sock manufacturing process may control the release of silver, The adsorption of the leached silver to WWTP biomass was used to develop a model which predicts that a typical wastewater treatment facility could treat a high concentration of influent silver. However, the high silver concentration may limit the disposal of the biosolids as agricultural fertilizer.
Document Type: Article
Addresses: [Benn, Troy M.; Westerhoff, Paul] Arizona State Univ, Tempe, AZ 85287 USA.
Reprint Address: Benn, TM (reprint author), Arizona State Univ, Box 5306, Tempe, AZ 85287 USA.
E-mail Addresses: Troy.Benn@asu.edu
Web of Science Categories: Engineering, Environmental; Environmental Sciences
ISSN: 0013-936X
Title: Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores
Author(s): Caskey, SR (Caskey, Stephen R.); Wong-Foy, AG (Wong-Foy, Antek G.); Matzger, AJ (Matzger, Adam J.)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 130 Issue: 33 Pages: 10870-+ DOI: 10.1021/ja8036096 Published: AUG 20 2008
Times Cited in Web of Science Core Collection: 1075
Total Times Cited: 1094
Abstract: A series of four isostructural microporous coordination polymers (MCPs) differing in metal composition is demonstrated to exhibit exceptional uptake of CO(2) at low pressures and ambient temperature. These conditions are particularly relevant for capture of flue gas from coal-fired power plants. A magnesium-based material is presented that is the highest surface area magnesium MCP yet reported and displays ultrahigh affinity based on heat of adsorption for CO(2). This study demonstrates that physisorptive materials can achieve affinities and capacities competitive with amine sorbents while greatly reducing the energy cost associated with regeneration.
Document Type: Article
Addresses: [Matzger, Adam J.] Univ Michigan, Dept Of Chem & Macromol Sci, Ann Arbor, MI 48109 USA.
Univ Michigan, Engn Program, Ann Arbor, MI 48109 USA.
Reprint Address: Matzger, AJ (reprint author), Univ Michigan, Dept Of Chem & Macromol Sci, 930 N Univ Ave, Ann Arbor, MI 48109 USA.
E-mail Addresses: matzger@umich.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: a thin-film rotating ring-disk electrode study
Author(s): Paulus, UA (Paulus, UA); Schmidt, TJ (Schmidt, TJ); Gasteiger, HA (Gasteiger, HA); Behm, RJ (Behm, RJ)
Source: JOURNAL OF ELECTROANALYTICAL CHEMISTRY Volume: 495 Issue: 2 Pages: 134-145 DOI: 10.1016/S0022-0728(00)00407-1 Published: JAN 5 2001
Times Cited in Web of Science Core Collection: 1073
Total Times Cited: 1086
Abstract: We describe the adaptation of the recently developed thin-film rotating disk electrode method and its application in a rotating ring disk configuration (RRDE) to the investigation of the oxygen reduction reaction (orr) on a supported catalyst powder (PtVulcan XC 72 carbon). This allows the determination of kinetic data, such as reaction orders or apparent activation energies, for the orr directly without mathematical modeling. Collection experiments reveal a potential and rotation rate independent collection efficiency. RRDE measurements allow. for the first time, the direct determination of the fraction of peroxide production juring oxygen reduction on supported catalysts. Finally, comparison of measurements in 0.5 M H2SO4 and 0.5 M HClO4, respectively, reveals a significant effect of (bi)sulfate adsorption on the orr activity. On the basis of the present results, predictions are made on the kinetic limit of the orr in polymer electrolyte fuel tells, in the absence of ohmic and mass transport resistances at 100% utilization. (C) 2001 Elsevier Science B.V. All rights reserved.
Document Type: Article
Addresses: Univ Ulm, Abt Oberflachenchem & Katalyse, D-89069 Ulm, Germany.
Reprint Address: Paulus, UA (reprint author), Paul Scherrer Inst, CH-5232 Villigen, Switzerland.
Web of Science Categories: Chemistry, Analytical; Electrochemistry
ISSN: 0022-0728
Title: Hydrogen sorption in functionalized metal-organic frameworks
Author(s): Rowsell, JLC (Rowsell, JLC); Millward, AR (Millward, AR); Park, KS (Park, KS); Yaghi, OM (Yaghi, OM)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 126 Issue: 18 Pages: 5666-5667 DOI: 10.1021/ja049408c Published: MAY 12 2004
Times Cited in Web of Science Core Collection: 1071
Total Times Cited: 1090
Document Type: Article
Addresses: Univ Michigan, Dept Chem, Ann Arbor, MI 48109 USA.
Reprint Address: Yaghi, OM (reprint author), Univ Michigan, Dept Chem, 930 N Univ Ave, Ann Arbor, MI 48109 USA.
E-mail Addresses: oyaghi@umich.edu
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: 'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption
Author(s): Gref, R (Gref, R); Luck, M (Luck, M); Quellec, P (Quellec, P); Marchand, M (Marchand, M); Dellacherie, E (Dellacherie, E); Harnisch, S (Harnisch, S); Blunk, T (Blunk, T); Muller, RH (Muller, RH)
Source: COLLOIDS AND SURFACES B-BIOINTERFACES Volume: 18 Issue: 3-4 Pages: 301-313 DOI: 10.1016/S0927-7765(99)00156-3 Published: OCT 2000
Times Cited in Web of Science Core Collection: 1070
Total Times Cited: 1103
Abstract: Nanoparticles possessing poly(ethylene glycol) (PEG) chains on their surface have been described as blood persistent drug delivery system with potential applications for intravenous drug administration. Considering the importance of protein interactions with injected colloidal dug carriers with regard to their in vivo fate, we analysed plasma protein adsorption onto biodegradable PEG-coated poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and poly(epsilon-caprolactone) (PCL) nanoparticles employing two-dimensional gel electrophoresis (2-D PAGE). A series of corona/core nanoparticles of sizes 160-270 nm were prepared from diblock PEG-PLA, PEG-PLGA and PEG-PCL and from PEG-PLA:PLA blends. The PEG Mw was varied from 2000-20 000 g/mole and the particles were prepared using different PEG contents. It was thus possible to study the influence of the PEG corona thickness and density, as well as the influence of the nature of the core (PLA, PLGA or PCL), on the competitive plasma protein adsorption, zeta potential and particle uptake by polymorphonuclear (PMN) cells. 2-D PAGE studies showed that plasma protein adsorption on PEG-coated PLA nanospheres strongly depends on the PEG molecular weight (Mw) (i.e. PEG chain length at the particle surface) as well as on the PEG content in the particles (i.e. PEG chain density at the surface of the particles). Whatever the thickness or the density of the corona, the qualitative composition of the plasma protein adsorption patterns was very similar, showing that adsorption was governed by interaction with a PLA surface protected more or less by PEG chains. The main spots on the gels were albumin, fibrinogen, IgG, Ig light chains, and the apolipoproteins apoA-I and apoE. For particles made of PEG-PLA45K with different PEG Mw, a maximal reduction in protein adsorption was found for a PEG Mw of 5000 g/mole. For nanospheres differing in their PEG content from 0.5 to 20 wt %, a PEG content between 2 and 5 wt % was determined as a threshold value for optimal protein resistance. When increasing the PEG content in the nanoparticles above 5 wt % no further reduction in protein adsorption was achieved. Phagocytosis by PMN studied using chemiluminescence and zeta potential data agreed well with these findings: the same PEG surface density threshold was found to ensure simultaneously efficient steric stabilization and to avoid the uptake by PMN cells. Supposing all the PEG chains migrate to the surface, this would correspond to a distance of about 1.5 nm between two terminally attached PEG chains in the covering 'brush'. Particles from PEG5K-PLA45K, PEG5K-PLGA45K; and PEG5K-PCL45K copolymers enabled to study the influence of the core on plasma protein adsorption, all other parameters (corona thickness and density) being kept constant. Adsorption patterns were in good qualitative agreement with each other. Only a few protein species were exclusively present just on one type of nanoparticle. However, the extent of proteins adsorbed differed in a large extent from one particle to another. In vivo studies could help elucidating the role of the type and amount of proteins adsorbed on the fate of the nanoparticles after intraveinous administration, as a function of the nature of their core. These results could be useful in the design of long circulating intravenously injectable biodegradable drug carriers endowed with protein resistant properties and low phagocytic uptake. (C) 2000 Elsevier Science B.V. All rights reserved.
Document Type: Article
Addresses: Univ Paris 11, Ctr Etud Pharmaceut, UMR CNRS 8612, Chatenay Malabry, France.
Free Univ Berlin, Dept Pharmaceut Biopharmaceut & Biotechnol, D-1000 Berlin, Germany.
ENSIC, UMR 7568, Lab Chim Phys Macromol, Nancy, France.
Etablissement Transfus Sanguine, Nancy, France.
MIT, Cambridge, MA 02139 USA.
Web of Science Categories: Biophysics; Chemistry, Physical; Materials Science, Biomaterials
ISSN: 0927-7765
Title: Mesoporous sieves with unified hybrid inorganic/organic frameworks
Author(s): Melde, BJ (Melde, BJ); Holland, BT (Holland, BT); Blanford, CF (Blanford, CF); Stein, A (Stein, A)
Source: CHEMISTRY OF MATERIALS Volume: 11 Issue: 11 Pages: 3302-3308 DOI: 10.1021/cm9903935 Published: NOV 1999
Times Cited in Web of Science Core Collection: 1067
Total Times Cited: 1084
Abstract: Mesoporous materials have been synthesized that are composed of hybrid frameworks in which inorganic and organic components have a fixed stoichiometry and are covalently bonded. The creation of UOFMN (unified organically functionalized mesoporous networks) materials incorporates concepts employed in the synthesis of MCM-41 mesoporous silicates, making use of a quaternary ammonium cationic surfactant and a double trialkoxysilyl precursor such as bis(triethoxysilyl)ethane (BTSE) or bis(triethoxysilyl)ethylene (BTSEY). The cetyltrimethylammonium (CTA(+)) surfactant is removed by extraction with acid, resulting in a high surface area porous organosilicate framework in which Si atoms are bridged by ethane (from BTSE) or ethylene (BTSEY) groups. The channels are wormlike and uniform in diameter. UOFMN materials are more hydrothermally stable than MCM-41 prepared under similar conditions and have thicker pore walls. Ethylene groups in products made with BTSEY can be brominated, the brominated product itself being reactive as a bromide source. The UOFMN products were characterized by XRD, N-2 adsorption, solid-state Si-29 and C-13 NMR, and TEM.
Document Type: Article
Addresses: Univ Minnesota, Dept Chem, Minneapolis, MN 55455 USA.
Reprint Address: Stein, A (reprint author), Univ Minnesota, Dept Chem, 207 Pleasant St SE, Minneapolis, MN 55455 USA.
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0897-4756
Title: Kinetic models of sorption: a theoretical analysis
Author(s): Azizian, S (Azizian, S)
Source: JOURNAL OF COLLOID AND INTERFACE SCIENCE Volume: 276 Issue: 1 Pages: 47-52 DOI: 10.1016/j.jcis.2004.03.048 Published: AUG 1 2004
Times Cited in Web of Science Core Collection: 1065
Total Times Cited: 1107
Abstract: The kinetics of sorption from a solution onto an adsorbent has been explored theoretically. The general analytical solution was obtained for two cases. It has been shown that at high initial concentration of solute (sorbate) the general equation converts to a pseudo-first-order model and at lower initial concentration of solute it converts to a pseudo-second-order model. In other words, the sorption process obeys pseudo-first-order kinetics at high initial concentration of solute, while it obeys pseudo-second-order kinetics model at lower initial concentration of solute. The theoretical results (derived equations) show that the observed rate constants of pseudo-first-order and pseudo-second-order models are combinations of adsorption and desorption rate constants and also initial concentration of solute. The obtained theoretical equations are used to correlate experimental data for sorption kinetics of some solutes on various sorbents. The predictions of the theory are in excellent agreement with the experimental data. (C) 2004 Elsevier Inc. All rights reserved.
Document Type: Article
Addresses: Bu Ali Sina Univ, Fac Sci, Dept Chem, Hamadan, Iran.
Reprint Address: Azizian, S (reprint author), Bu Ali Sina Univ, Fac Sci, Dept Chem, Hamadan, Iran.
E-mail Addresses: sazizian@basu.ac.ir
Web of Science Categories: Chemistry, Physical
ISSN: 0021-9797
Title: Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs
Author(s): Wang, B (Wang, Bo); Cote, AP (Cote, Adrien P.); Furukawa, H (Furukawa, Hiroyasu); O'Keeffe, M (O'Keeffe, Michael); Yaghi, OM (Yaghi, Omar M.)
Source: NATURE Volume: 453 Issue: 7192 Pages: 207-U6 DOI: 10.1038/nature06900 Published: MAY 8 2008
Times Cited in Web of Science Core Collection: 1048
Total Times Cited: 1067
Abstract: Zeolitic imidazolate frameworks ( ZIFs) are porous crystalline materials with tetrahedral networks that resemble those of zeolites: transition metals ( Zn, Co) replace tetrahedrally coordinated atoms ( for example, Si), and imidazolate links replace oxygen bridges(1). A striking feature of these materials is that the structure adopted by a given ZIF is determined by link - link interactions, rather than by the structure directing agents used in zeolite synthesis(2). As a result, systematic variations of linker substituents have yielded many different ZIFs that exhibit known or predicted zeolite topologies. The materials are chemically and thermally stable, yet have the long- sought- after design flexibility offered by functionalized organic links and a high density of transition metal ions(1-7) . Here we report the synthesis and characterization of two porous ZIFs-ZIF-95 and ZIF-100-with structures of a scale and complexity previously unknown in zeolites(8-10). The materials have complex cages that contain up to 264 vertices, and are constructed from as many as 7,524 atoms. As expected from the adsorption selectivity recently documented for other members of this materials family(3), both ZIFs selectively capture carbon dioxide from several different gas mixtures at room temperature, with ZIF-100 capable of storing 28 litres per litre of material at standard temperature and pressure. These characteristics, combined with their high thermal and chemical stability and ease of fabrication, make ZIFs promising candidate materials for strategies aimed at ameliorating increasing atmospheric carbon dioxide levels.
Document Type: Article
Addresses: [Wang, Bo; Cote, Adrien P.; Furukawa, Hiroyasu; Yaghi, Omar M.] Univ Calif Los Angeles, Dept Chem & Biochem, Ctr Reticular Chem, Los Angeles, CA 90095 USA.
[O'Keeffe, Michael] Arizona State Univ, Dept Chem & Biochem, Tempe, AZ 85287 USA.
Reprint Address: Yaghi, OM (reprint author), Univ Calif Los Angeles, Dept Chem & Biochem, Ctr Reticular Chem, 607 E Charles E Young Dr, Los Angeles, CA 90095 USA.
E-mail Addresses: yaghi@chem.ucla.edu
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks
Author(s): Zhao, XB (Zhao, XB); Xiao, B (Xiao, B); Fletcher, AJ (Fletcher, AJ); Thomas, KM (Thomas, KM); Bradshaw, D (Bradshaw, D); Rosseinsky, MJ (Rosseinsky, MJ)
Source: SCIENCE Volume: 306 Issue: 5698 Pages: 1012-1015 DOI: 10.1126/science.1101982 Published: NOV 5 2004
Times Cited in Web of Science Core Collection: 1047
Total Times Cited: 1055
Abstract: Adsorption and desorption of hydrogen from nanoporous materials, such as activated carbon, is usually fully reversible. We have prepared nanoporous metal-organic framework materials with flexible linkers in which the pore openings, as characterized in the static structures, appear to be too small to allow H-2 to pass. We observe hysteresis in their adsorption and desorption kinetics above the supercritical temperature of H-2 that reflects the dynamical opening of the "windows" between pores. This behavior would allow H-2 to be adsorbed at high pressures but stored at lower pressures.
Document Type: Article
Addresses: Univ Newcastle Upon Tyne, Sch Nat Sci, No Carbon Res Labs, Newcastle Upon Tyne NE1 7RU, Tyne & Wear, England.
Univ Liverpool, Dept Chem, Liverpool, Merseyside, England.
Reprint Address: Thomas, KM (reprint author), Univ Newcastle Upon Tyne, Sch Nat Sci, No Carbon Res Labs, Newcastle Upon Tyne NE1 7RU, Tyne & Wear, England.
E-mail Addresses: mark.thomas@ncl.ac.uk
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0036-8075
eISSN: 1095-9203
Title: Use of cellulose-based wastes for adsorption of dyes from aqueous solutions
Author(s): Annadurai, G (Annadurai, G); Juang, RS (Juang, RS); Lee, DJ (Lee, DJ)
Source: JOURNAL OF HAZARDOUS MATERIALS Volume: 92 Issue: 3 Pages: 263-274 Article Number: PII S0304-3894(02)00017-1 DOI: 10.1016/S0304-3894(02)00017-1 Published: JUN 10 2002
Times Cited in Web of Science Core Collection: 1046
Total Times Cited: 1093
Abstract: Low-cost banana and orange peels were prepared as adsorbents for the adsorption of dyes from aqueous solutions. Dye concentration and pH were varied. The adsorption capacities for both peels decreased in the order methyl orange (MO) > methylene blue (MB) > Rhodamine B (RB) > Congo red (CR) > methyl violet (MV) > amido black 10B (AB). The isotherm data could be well described by the Freundlich and Langmuir equations in the concentration range of 10-120 mg/l. An alkaline pH was favorable for the adsorption of dyes. Based on the adsorption capacity, it was shown that banana peel was more effective than orange peel. Kinetic parameters of adsorption such as the Langergren rate constant and the intraparticle diffusion rate constant were determined. For the present adsorption process intraparticle diffusion of dyes within the particle was identified to be rate limiting. Both peel wastes were shown to be promising materials for adsorption removal of dyes from aqueous solutions. (C) 2002 Elsevier Science B.V. All rights reserved.
Document Type: Article
Addresses: Yuan Ze Univ, Dept Chem Engn, Chungli 320, Taiwan.
Natl Taiwan Univ, Dept Chem Engn, Taipei 106, Taiwan.
Reprint Address: Juang, RS (reprint author), Yuan Ze Univ, Dept Chem Engn, Chungli 320, Taiwan.
Web of Science Categories: Engineering, Environmental; Environmental Sciences
ISSN: 0304-3894
Title: Highly controlled acetylene accommodation in a metal-organic microporous material
Author(s): Matsuda, R (Matsuda, R); Kitaura, R (Kitaura, R); Kitagawa, S (Kitagawa, S); Kubota, Y (Kubota, Y); Belosludov, RV (Belosludov, RV); Kobayashi, TC (Kobayashi, TC); Sakamoto, H (Sakamoto, H); Chiba, T (Chiba, T); Takata, M (Takata, M); Kawazoe, Y (Kawazoe, Y); Mita, Y (Mita, Y)
Source: NATURE Volume: 436 Issue: 7048 Pages: 238-241 DOI: 10.1038/nature03852 Published: JUL 14 2005
Times Cited in Web of Science Core Collection: 1037
Total Times Cited: 1047
Abstract: Metal - organic microporous materials(1-4) (MOMs) have attracted wide scientific attention owing to their unusual structure and properties, as well as commercial interest due to their potential applications in storage(5-9), separation(10,11) and heterogeneous catalysis(12,13). One of the advantages of MOMs compared to other microporous materials, such as activated carbons, is their ability to exhibit a variety of pore surface properties such as hydrophilicity and chirality, as a result of the controlled incorporation of organic functional groups into the pore walls(11,13-15). This capability means that the pore surfaces of MOMs could be designed to adsorb specific molecules; but few design strategies for the adsorption of small molecules have been established so far. Here we report high levels of selective sorption of acetylene molecules as compared to a very similar molecule, carbon dioxide, onto the functionalized surface of a MOM. The acetylene molecules are held at a periodic distance from one another by hydrogen bonding between two non-coordinated oxygen atoms in the nanoscale pore wall of the MOM and the two hydrogen atoms of the acetylene molecule. This permits the stable storage of acetylene at a density 200 times the safe compression limit of free acetylene at room temperature.
Document Type: Article
Addresses: Kyoto Univ, Grad Sch Engn, Dept Synthet Chem & Biol Chem, Kyoto 6158510, Japan.
Osaka Prefecture Univ, Grad Sch Sci, Dept Phys Sci, Osaka 5900035, Japan.
Tohoku Univ, Inst Mat Res, Sendai, Miyagi 9808577, Japan.
Okayama Univ, Fac Sci, Dept Phys, Okayama 7008530, Japan.
Japan Synchrotron Radiat Res Inst SPring8, Sayo, Hyogo 6795198, Japan.
Japan Sci & Technol Agcy, CREST, Tokyo, Japan.
Osaka Univ, Grad Sch Engn Sci, Dept Mat Engn, Div Mat Phys, Osaka 5608531, Japan.
Reprint Address: Kitagawa, S (reprint author), Kyoto Univ, Grad Sch Engn, Dept Synthet Chem & Biol Chem, Kyoto 6158510, Japan.
E-mail Addresses: kitagawa@sbchem.kyoto-u.ac.jp
Web of Science Categories: Multidisciplinary Sciences
ISSN: 0028-0836
Title: ELLIPSOMETRY AS A TOOL TO STUDY ADSORPTION BEHAVIOR OF SYNTHETIC AND BIOPOLYMERS AT AIR-WATER-INTERFACE
Author(s): DEFEIJTER, JA (DEFEIJTER, JA); BENJAMINS, J (BENJAMINS, J); VEER, FA (VEER, FA)
Source: BIOPOLYMERS Volume: 17 Issue: 7 Pages: 1759-1772 DOI: 10.1002/bip.1978.360170711 Published: 1978
Times Cited in Web of Science Core Collection: 1035
Total Times Cited: 1038
Document Type: Article
Reprint Address: DEFEIJTER, JA (reprint author), UNILEVER RES LAB,VLAARDINGEN,NETHERLANDS.
Web of Science Categories: Biochemistry & Molecular Biology; Biophysics
ISSN: 0006-3525
Title: Characterization of the porous structure of SBA-15
Author(s): Kruk, M (Kruk, M); Jaroniec, M (Jaroniec, M); Ko, CH (Ko, CH); Ryoo, R (Ryoo, R)
Source: CHEMISTRY OF MATERIALS Volume: 12 Issue: 7 Pages: 1961-1968 DOI: 10.1021/cm000164e Published: JUL 2000
Times Cited in Web of Science Core Collection: 1018
Total Times Cited: 1050
Abstract: SBA-15 ordered mesoporous silicas were synthesized using the method reported by Zhao et al, The structures of these materials were characterized using powder X-ray diffraction (XRD), thermogravimetric analysis (TGA), and nitrogen adsorption. The samples were found to exhibit structural properties similar to those reported earlier. Our study confirmed that the size of primary mesopores of SBA-15 can be tailored by the choice of synthesis temperature and that SBA-15 exhibits a significant amount of disordered micropores and small mesopores, The volume and size of these complementary pores were found to be dependent to some extent on the synthesis/aging temperature. It was shown that the washing of as-synthesized SBA-15 in water or ethanol was accompanied by an appreciable structural shrinkage and led to the removal of a significant part of the polymeric template. Therefore, washing needs to be avoided if one wants to isolate SBA-15 without appreciable loss of the template. It was confirmed that water-washed SBA-15 samples have fully accessible primary mesopores. Ethanol-washed samples also were found to exhibit accessible porosity. Despite an appreciable content of the template in the water- and ethanol-washed samples, their pore sizes were usually larger than those of the calcined materials. The observed structural properties of SBA-15 and their dependence on the synthesis temperature and washing were attributed to the changes in the degree of penetration of the poly(ethylene oxide) chains of the triblock copolymer template within the siliceous walls of SBA-15.
Document Type: Article
Addresses: Kent State Univ, Dept Chem, Kent, OH 44242 USA.
Korea Adv Inst Sci & Technol, Dept Chem, Taejon 305701, South Korea.
Korea Adv Inst Sci & Technol, Sch Mol Sci BK21, Taejon 305701, South Korea.
Reprint Address: Jaroniec, M (reprint author), Kent State Univ, Dept Chem, Kent, OH 44242 USA.
E-mail Addresses: Jaroniec@columbo.kent.edu
Web of Science Categories: Chemistry, Physical; Materials Science, Multidisciplinary
ISSN: 0897-4756
Title: Flexible porous metal-organic frameworks for a controlled drug delivery
Author(s): Horcajada, P (Horcajada, Patricia); Serre, C (Serre, Christian); Maurin, G (Maurin, Guillaume); Ramsahye, NA (Ramsahye, Naseem A.); Balas, F (Balas, Francisco); Vallet-Regi, M (Vallet-Regi, Maria); Sebban, M (Sebban, Muriel); Taulelle, F (Taulelle, Francis); Ferey, G (Ferey, Gerard)
Source: JOURNAL OF THE AMERICAN CHEMICAL SOCIETY Volume: 130 Issue: 21 Pages: 6774-6780 DOI: 10.1021/ja710973k Published: MAY 28 2008
Times Cited in Web of Science Core Collection: 1012
Total Times Cited: 1028
Abstract: Flexible nanoporous chromium or iron terephtalates (BDC) MIL-53(Cr, Fe) or M(OH)[BDC] have been used as matrices for the adsorption and in vitro drug delivery of lbuprofen (or alpha-p-isobutylphenylpropionic acid). Both MIL-53(Cr) and MIL-53(Fe) solids adsorb around 20 wt% of lbuprofen (lbuprofen/dehydrated MIL-53 molar ratio = 0.22(1)), indicating that the amount of inserted drug does not depend on the metal (Cr, Fe) constitutive of the hybrid framework. Structural and spectroscopic characterizations are provided for the solid filled with lbuprofen. In each case, the very slow and complete delivery of lbuprofen was achieved under physiological conditions after 3 weeks with a predictable zero-order kinetics, which highlights the unique properties of flexible hybrid solids for adapting their pore opening to optimize the drug-matrix interactions.
Document Type: Article
Addresses: [Horcajada, Patricia; Balas, Francisco; Vallet-Regi, Maria] Univ Complutense Madrid, Dept Quim Inorgan & Bioinorgan, Fac Farm, E-28040 Madrid, Spain.
[Maurin, Guillaume; Ramsahye, Naseem A.] Univ Montpellier 2, Inst Charles Gerhardt, CNRS, UMR 5253,UM2,ENSCM, F-34095 Montpellier 5, France.
[Ferey, Gerard] Inst Univ France, Paris, France.
[Horcajada, Patricia; Serre, Christian; Taulelle, Francis; Ferey, Gerard] Univ Versailles St Quentin Yvelines, CNRS, UMR 8180, Inst Lavoisier, F-78035 Versailles, France.
Reprint Address: Serre, C (reprint author), Univ Versailles St Quentin Yvelines, CNRS, UMR 8180, Inst Lavoisier, 45 Ave Etats Unis, F-78035 Versailles, France.
E-mail Addresses: serre@chimie.uvsq.fr
Web of Science Categories: Chemistry, Multidisciplinary
ISSN: 0002-7863
Title: Arsenite and arsenate adsorption on ferrihydrite: Kinetics, equilibrium, and adsorption envelopes
Author(s): Raven, KP (Raven, KP); Jain, A (Jain, A); Loeppert, RH (Loeppert, RH)
Source: ENVIRONMENTAL SCIENCE & TECHNOLOGY Volume: 32 Issue: 3 Pages: 344-349 DOI: 10.1021/es970421p Published: FEB 1 1998
Times Cited in Web of Science Core Collection: 1004
Total Times Cited: 1051
Abstract: Because of its toxicity, arsenic is of considerable environmental concern. Its solubility in natural systems is strongly influenced by adsorption at iron oxide surfaces. The objective of this study was to compare the adsorption behavior of arsenite and arsenate on ferrihydrite, under carefully controlled conditions, with regard to adsorption kinetics, adsorption isotherms, and the influence of pH on adsorption. The adsorption reactions were relatively fast, with the reactions almost completed within the first few hours. At relatively high As concentrations, arsenite reacted faster than arsenate with the ferrihydrite, i.e., equilibrium was achieved sooner, but arsenate adsorption was faster at low As concentrations and low pH. Adsorption maxima of approximately 0.60 (0.58) and 0.25 (0.16) mol(As), mol(Fe)(-1) were achieved for arsenite and arsenate, respectively, at pH 4.6 (pH 9.2 in parentheses). The high arsenite retention, which precludes its retention entirely as surface adsorbed species, indicates the likelihood that ferrihydrite was transformed to a ferric arsenite phase, although this possibility has yet to be confirmed by spectroscopic studies. The general trend at initial arsenic concentrations greater than or equal to 0.27 mol(As) kg(-1) ferrihydrite within the pH range of 4-9 was increasing arsenite adsorption and decreasing arsenate adsorption with increasing pH. At initial As concentrations of 0.27-0.80 mol(As), kg(-1) ferrihydrite, the adsorption envelopes crossed at approximately pH 6-7.5, i.e., adsorbed arsenate concentrations were relatively greater than adsorbed arsenite concentrations at lower pH values whereas adsorbed arsenite was greater at higher pH. At the highest initial arsenic concentration of 13.3 mol(As) kg(-1) ferrihydrite, a distinct adsorption maximum was observed for arsenite adsorption at approximately pH 9.0, which corresponds closely to the first pK(a) (9.2) of H3AsO30, whereas there was a continuous drop in arsenate adsorption with increasing pH from 3 to 11.
Document Type: Article
Addresses: Texas A&M Univ, Dept Soil & Crop Sci, College Stn, TX 77843 USA.
Reprint Address: Loeppert, RH (reprint author), Texas A&M Univ, Dept Soil & Crop Sci, College Stn, TX 77843 USA.
Web of Science Categories: Engineering, Environmental; Environmental Sciences
ISSN: 0013-936X






















![[Insert Picture Here]](1000%20WC.files/image028.jpg)























![[Insert Picture Here]](1000%20WC.files/image052.jpg)


















![[Insert Picture Here]](1000%20WC.files/image071.jpg)







